Adial (ADIL) Shares Up 43% in the Past Week: Here's Why
In the past week, shares of Adial Pharmaceuticals ADIL have gained 43.4% compared with the industry’s 1.6% rise.
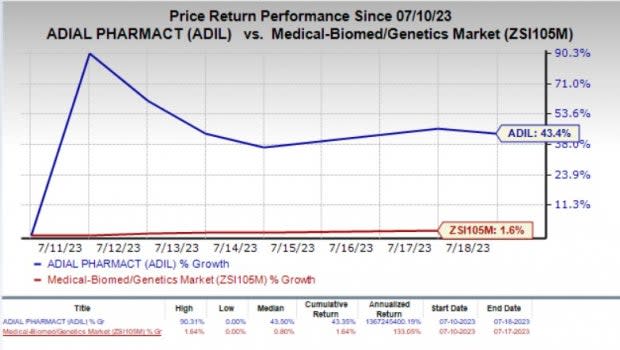
Image Source: Zacks Investment Research
The steep rise in the stock price was observed after the company provided a summary of positive feedback received from respective regulatory bodies in the United States and EU regarding Adial’s propped phase III confirmatory study for AD04 in treating alcohol use disorder (AUD). Additionally, based on the feedback received, ADIL also elaborated on its current clinical development plan for AD04.
AD04 is a genetically targeted, serotonin-3 receptor antagonist, which is currently under development for treating patients with AUD. The positive feedback from the regulatory bodies signifies agreement regarding the importance of ongoing research in the AUD therapeutic area as a persistent high unmet need.
An agreement was also reached regarding the primary endpoint of the confirmatory study based on the percentage of no heavy drinking days. This primary endpoint will be determined by making use of a responder analysis of patients who reduced their alcohol consumption to zero heavy drinking days in the last two months of a six-month study.
The regulatory bodies also acknowledged the promising nature of Adial’s post hoc analysis results from its phase II and phase III studies of AD04 in the treatment of AUD. It was observed that patients with a specific genetic subtype achieved statistical significance of responder analysis in both the phase II and phase III studies. Additionally, patients averaging more than 17 heavy drinking days per month at the start of the study achieved under three average heavy drinking days per month at study completion.
These post hoc analysis data reportedly played a pivotal role in planning future studies of AD04. The safety profile of the drug in the late-stage ONWARD study of AD04 to treat AUD was also found to be acceptable by the authorities.
However, the regulatory authorities recommended that Adial identifies a patient subgroup where a relevant treatment effect and compelling evidence of a favorable risk-benefit profile can be assessed.
The feedback from the regulatory bodies also encompassed efficacy and safety requirements for the developmental candidate. The efficacy issue stated that the data from one single phase III confirmatory study may not be sufficient for approval of the drug candidate. Hence, Adial has agreed to conduct two studies of AD04 simultaneously. One of the studies will compare patients treated with AD04 against treatment with placebo. On the other hand, a second study may include a biomarker-negative patient arm to satisfy any ongoing questions from the regulators regarding efficacy parameters.
Per ADIL, conducting two simultaneous studies is the best strategy to minimize risk, optimize timing and costs, as well as improve the probability of regulatory authority acceptance and approval in the United Statues and Europe. The company is currently in the process of confirming its clinical development plan and pathwayto satisfy both the U.S. and EU AD04 submission requirements.
Adial also plans to add a long-term safety follow-up to the planned phase III study, which will expose more than 100 patients to treat with AD04 for a year. This will provide the company with sufficient safety data to satisfy the regulatory authorities.
Subject to the success of the two simultaneous studies, ADIL expects to file a new drug application with the FDA in the third quarter of 2025. Adial anticipates the total cost of the development plan around $25 million. The company is actively looking for potential commercial partners to support the planned late-stage studies of AD04 and advance its commercialization in both the U.S. and European markets.
Adial Pharmaceuticals, Inc. Price and Consensus
Adial Pharmaceuticals, Inc. price-consensus-chart | Adial Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Adial currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same industry are ADC Therapeutics ADCT, Acadia Pharmaceuticals ACAD and Akoya Biosciences AKYA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for ADC Therapeutics’ 2023 loss per share has widened from $2.58 to $2.61. During the same period, the estimate for ADC Therapeutics’ 2024 loss per share narrowed from $2.72 to $2.45. In the past week, shares of ADCT have lost 36.7%.
ADCT beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 10.70%.
In the past 90 days, the Zacks Consensus Estimate for Acadia Pharmaceuticals’ 2023 loss per share has narrowed from 57 cents to 33 cents. The estimate for Acadia Pharmaceuticals’ 2024 earnings per share is pegged at 21 cents. In the past week, shares of ACAD have risen 33.2%.
ACAD beat estimates in two of the trailing four quarters, missing the mark on other two occasions, delivering an average negative earnings surprise of 2.75%.
In the past 90 days, the Zacks Consensus Estimate for Akoya Biosciences’ 2023 loss per share has narrowed from $1.80 to $1.71. During the same period, the estimate for Akoya Biosciences’ 2024 loss per share narrowed from $1.57 to $1.33. In the past week, shares of AKYA have lost 1.1%.
AKYA missed estimates in each of the trailing four quarters, delivering an average negative earnings surprise of 21.05%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ADC Therapeutics SA (ADCT) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
Adial Pharmaceuticals, Inc. (ADIL) : Free Stock Analysis Report
Akoya Biosciences, Inc. (AKYA) : Free Stock Analysis Report
