Aeglea (AGLE) Submits BLA for a Rare Disease Drug to the FDA
Aeglea BioTherapeutics, Inc. AGLE announced that it has submitted a biologics license application (“BLA”) to the FDA, seeking approval for its lead product candidate, pegzilarginase for the treatment of arginase 1 deficiency (ARG1-D), a devastating rare disease.
The company had requested the FDA to grant priority review to the BLA.
If approved, pegzilarginase will become the first treatment for ARG1-D to be approved by the FDA.
The BLA filling was based on data from several clinical studies, including the phase III PEACE study, an ongoing long-term extension study, a phase I/II study as well as an open-label extension study that evaluated pegzilarginase for treating ARG1-D.
Data from these studies showed that treatment with pegzilarginase demonstrated rapidly and sustainably lower arginine levels, and showed improvements in mobility.
However, the company said that the FDA has given a feedback conveying its disagreement with the substantial evidence regarding the effectiveness of pegzilarginase. This hurt investors sentiments and resulted in the stock declining 8.2% on Tuesday in response.
The stock has plunged 53% so far this year compared with the industry’s decrease of 13.9%.
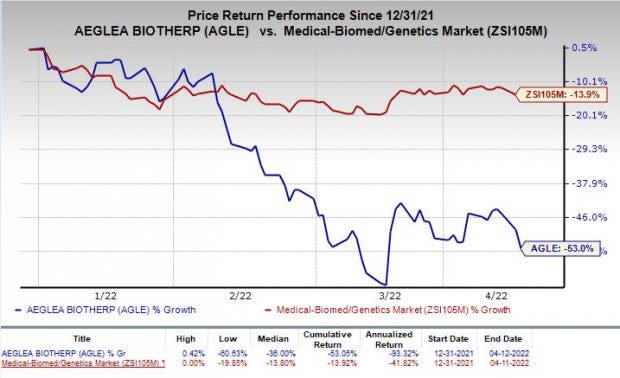
Image Source: Zacks Investment Research
However, based on the totality and compelling clinical data on pegzilarginase, Aeglea decided to go ahead with the BLA submission and provide the FDA access to all relevant information.
The FDA has granted Rare Pediatric Disease, Breakthrough Therapy, Fast Track and Orphan Drug designations to pegzilarginase for the treatment of ARG1-D.
Aeglea's commercialization partner in Europe and the Middle East, Immedica Pharma AB, plans to submit the marketing authorization application for pegzilarginase to the European Medicines Agency later in 2022.
Upon potential approval, pegzilarginase is likely to serve an area of high unmet medical need for the ARG1-D community.
Aeglea has no approved product in its portfolio at the moment. Therefore, the successful development of pegzilarginase, along with other pipeline candidates, remains a key focus for the company.
Zacks Rank & Stocks to Consider
Aeglea currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector are Aligos Therapeutics, Inc. ALGS, Applied Therapeutics, Inc. APLT and Voyager Therapeutics, Inc. VYGR, all carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Zacks Consensus Estimate for Aligos Therapeutics’ loss per share has narrowed 15.1% for 2022 and 45.7% for 2023 over the past 60 days.
Earnings of ALGS surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Applied Therapeutics’ loss per share estimates have narrowed 11.9% for 2022 and 15.7% for 2023 over the past 60 days.
Earnings of Applied Therapeutics have surpassed estimates in two of the trailing four quarters, met the same once and missed the same on the other occasion.
Voyager Therapeutics’ loss per share estimates have narrowed 38.6% for 2022 and 29% for 2023 over the past 60 days. The VYGR stock has skyrocketed 197.1% year to date.
Earnings of Voyager Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Voyager Therapeutics, Inc. (VYGR) : Free Stock Analysis Report
Aeglea BioTherapeutics, Inc. (AGLE) : Free Stock Analysis Report
Applied Therapeutics Inc. (APLT) : Free Stock Analysis Report
Aligos Therapeutics, Inc. (ALGS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
