Agios (AGIO) Meets Clinical Proof-of-Concept in Anemia Treatment
Agios Pharmaceuticals AGIO achieved clinical proof-of-concept in the phase IIa portion of a study evaluating its investigational next-generation pyruvate kinase (PK) activator AG-946 to treat anemia in adults with lower-risk myelodysplastic syndromes (MDS).
A total of 22 patients were enrolled in this phase IIa portion and classified into two cohorts — 12 classified as non-transfused (NTD) and 10 classified as low transfusion burden (LTB). The primary endpoints of the study were transfusion dependence (applicable only for the LTB cohort) and hemoglobin response.
Data from the phase IIa portion of the study revealed that 40% of patients in the LTB cohort achieved the transfusion independence endpoint. In contrast, one of the 22 patients treated in the study achieved the hemoglobin response endpoint in the 16-week treatment (core) period.
Per Agios, these results highlight the potential of AG-946 by improving red blood cell (RBC) health through its unique mechanism of action. A significant reduction in transfusions allows patients to potentially lower visits to the clinic, thereby improving their quality of life.
The safety profile of the drug was also consistent with the data reported in a previously conducted study on healthy volunteers. Management plans to present more data from the phase IIa portion at a medical meeting next year.
Based on these results, management plans to start the placebo-controlled phase IIb portion of the study in mid-2024, evaluating AG-946 in lower-risk MDS.
Year to date, shares of Agios have lost 23.4% compared with the industry’s 8.6% fall.
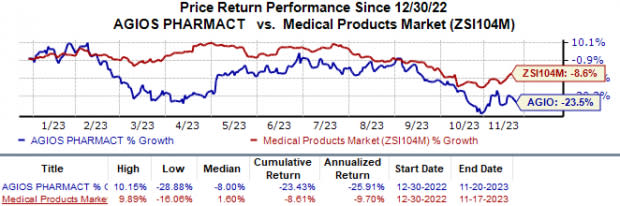
Image Source: Zacks Investment Research
AG-946 is the company’s second PK activator being evaluated for clinical studies. Last year, Agios received FDA approval for Pyrukynd (mitapivat), a first-in-class PK activator for treating hemolytic anemia in adults with PK deficiency. Currently, Agios’ sole marketed drug, Pyrukynd, is also being developed for other hemolytic anemias, including sickle cell disease and thalassemia, in several label expansion studies.
If successfully developed and commercialized, AG-946 is likely to face stiff competition from Bristol Myers’ BMY Reblozyl (luspatercept). The Bristol Myers drug recently received label expansion in the United States as the first-line treatment of anemia in adults with lower-risk MDS who may require transfusions.
Bristol Myers’ Reblozyl was already approved by the FDA to treat anemia in adult patients with beta-thalassemia who require regular RBC transfusions. It is also approved for the treatment of anemia failing an erythropoiesis-stimulating agent and requiring two or more RBC units over eight weeks in adult patients with very-low-to-intermediate-risk MDS with ring sideroblasts (MDS-RS) or with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis.
Agios Pharmaceuticals, Inc. Price
Agios Pharmaceuticals, Inc. price | Agios Pharmaceuticals, Inc. Quote
Zacks Rank & Stocks to Consider
Agios currently carries a Zacks Rank #3 (Hold).Some better-ranked stocks in the overall healthcare sector include Acadia Pharmaceuticals ACAD and AnaptysBio ANAB, all carrying a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Acadia Pharmaceuticals’ loss estimates for 2023 have narrowed from 41 cents to 34 cents per share in the past 60 days. During the same period, the estimates for 2024 earnings per share have risen from 52 cents to 90 cents. Year to date, Acadia Pharmaceuticals’ shares have gained 44.0%.
Acadia Pharmaceuticals beat earnings estimates in two of the last four quarters while missing the mark on the other two occasions, witnessing an earnings surprise of 20.69% on average. In the last reported quarter, ACAD reported an earnings surprise of 6.98%.
AnaptysBio’s loss estimate has narrowed from $6.57 to $6.08 per share in the past 60 days. During the same period, the loss estimates per share for 2024 have narrowed from $6.93 to $6.38. Shares of ANAB have lost 53.2% in the year-to-date period.
The earnings of AnaptysBio beat estimates in two of the last four quarters while missing the mark on the other two occasions, posting a negative average earnings surprise of 6.48%. AnaptysBio’s earnings beat estimates by 18.02%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Agios Pharmaceuticals, Inc. (AGIO) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
AnaptysBio, Inc. (ANAB) : Free Stock Analysis Report
