Alnylam (ALNY) Announces FDA Committee Onpattro sNDA Review Date
Alnylam Pharmaceuticals ALNY announced that the FDA’s Cardiovascular and Renal Drugs Advisory Committee is scheduled to meet on Sep 13, 2023, to review the supplemental new drug application (sNDA) for patisiran in the treatment for the cardiomyopathy of transthyretin-mediated (ATTR) amyloidosis.
A final decision from the FDA is expected on Oct 8, 2023.
Patisiran is currently marketed as Onpattro in the United States and Europe for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. Onpattro is the first and only FDA-approved treatment for this indication.
Year to date, shares of Alnylam have declined 20% compared with the industry’s 10.1% fall.
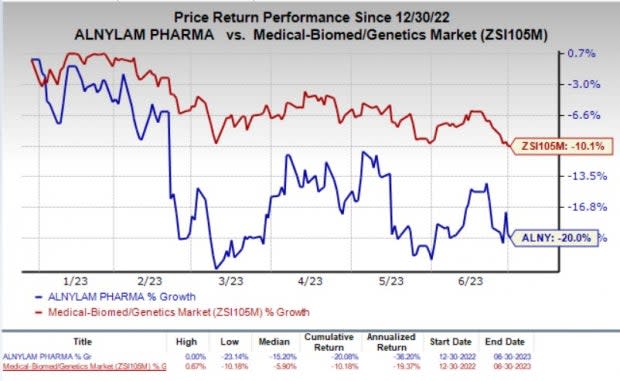
Image Source: Zacks Investment Research
We would like to remind the investors that Alnylam filed the sNDA in December 2022 to expand the label of Onpattro in ATTR amyloidosis patients with cardiomyopathy. The sNDA application was based on positive results from the APOLLO-B study.
In September 2022, ALNY reported that the phase III APOLLO-B study, evaluating patisiran for the treatment of ATTR amyloidosis with cardiomyopathy, met its primary endpoint of change from baseline in the 6-Minute Walk Test versus placebo at 12 months. The study also met the first secondary endpoint of change from baseline in quality of life versus placebo.
Furthermore, in May 2023, the company announced new positive results from an interim analysis of exploratory data from the open-label extension (OLE) period of the phase III APOLLO-B study of patisiran for the treatment of ATTR amyloidosis with cardiomyopathy.
The interim analysis was carried out on the 18-month data of the APOLLO-B study. Results indicated that the previously achieved primary endpoint, health status and quality of life, observed at the end of 12 months of the same study, were sustained with continued treatment with patisiran during the OLE period (at the end of 18 months).
Alnylam has reportedly submitted the 18-month data from the interim analysis of the OLE period to the FDA as an amendment to the sNDA for patisiran for the treatment of the cardiomyopathy of ATTR amyloidosis.
Currently, Onpattro revenues are witnessing a steady decline as more and more patients are shifting to another drug in Alnylam’s portfolio, Amvuttra (vutrisiran). Amvuttra received FDA approval in June 2022, for the treatment of adult patients with polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis. Amvuttra is also approved in the EU for the treatment of hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy.
Alnylam Pharmaceuticals, Inc. Price and Consensus
Alnylam Pharmaceuticals, Inc. price-consensus-chart | Alnylam Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Alnylam currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Adaptimmune Therapeutics ADAP, Akero Therapeutics AKRO and ADMA Biologics, Inc. ADMA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Adaptimmune Therapeutics’ 2023 loss per share has narrowed from 63 cents to 46 cents. During the same period, the estimate for Adaptimmune Therapeutics’ 2024 loss per share has narrowed from 59 cents to 56 cents. Year to date, shares of ADAP have fallen by 36.7%.
ADAP beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 36.89%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $2.96 to $2.80. During the same period, the estimate for Akero Therapeutics’ 2024 loss per share has narrowed from $3.40 to $3.27. Year to date, shares of AKRO have lost 14.8%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
In the past 90 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 14 cents to 9 cents. The consensus estimate for 2024 earnings is currently pegged at 7 cents per share. Year to date, shares of ADMA have lost 4.9%.
ADMA beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 19.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Adaptimmune Therapeutics PLC (ADAP) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
