Alnylam (ALNY) Q2 Loss Wider Than Expected, Revenues Miss
Alnylam Pharmaceuticals, Inc. ALNY incurred a loss of $2.21 per share in the second quarter of 2023, wider than the Zacks Consensus Estimate of a loss of $1.72 per share.
The second-quarter loss included stock-based compensation expenses and realized/unrealized gain on marketable equity securities. Excluding these items, the adjusted loss was $1.62 per share, narrower than the adjusted loss of $2.03 reported in the year-ago quarter.
Alnylam recorded total revenues of $319 million in the quarter, up 41.8% from the year-ago quarter, but missed the Zacks Consensus Estimate of $332 million. Net product revenues were $305.7 million, up 43% year over year on a reported basis and 44% at constant exchange rate (CER), driven by increased patient demand for the newly approved drug, Amvuttra (vutrisiran), along with Givlaari (givosiran) and Oxlumo (lumasiran).
Net revenues from collaborators were $5.8 million, down 35.2% from $9 million in the year-ago quarter. The decline was due to operating variability, including the level of work reimbursed in Alnylam’s collaboration with Regeneron Pharmaceuticals, Inc. REGN.
In 2019, Alnylam and Regeneron entered into a global and strategic collaboration agreement to co-develop and co-commercialize RNAi therapeutics for a broad range of eye and central nervous system diseases. Following the initial agreement, in July 2020, Regeneron exercised its co-development/co-commercialization option on Alnylam’s first central nervous system targeted development candidate, ALN-APP.
Alnylam earned collaboration revenues of $8.6 million in the reported quarter from Novartis NVS. Novartis has exclusive and worldwide rights to manufacture and commercialize RNAi therapeutics targeting PCSK9 for the treatment of hypercholesterolemia and other human diseases, including Leqvio (inclisiran).
During the reported quarter, Novartis reported that the FDA has extended Leqvio’s label to include earlier use in patients with elevated LDL-C who have an increased risk of heart disease, as an adjunct to diet and statin therapy.
Alnylam is entitled to receive license fees from these collaboration agreements.
During the second quarter, ALNY recorded royalty revenues of $7.2 million, primarily due to increased royalties from third parties driven by sales of Amvuttra. In the year-ago quarter, the company recorded royalty revenues of $2.3 million.
Shares of Alnylam have plunged 20% year to date compared with the industry’s decline of 12.9%.
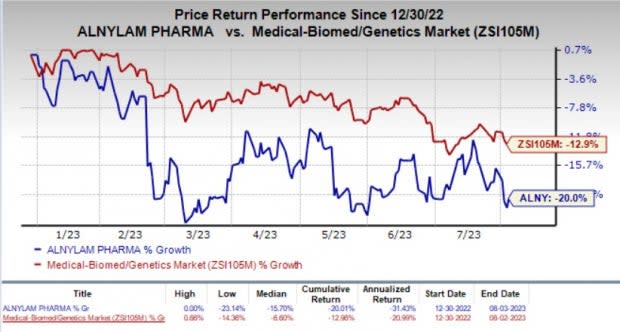
Image Source: Zacks Investment Research
Quarter in Detail
Onpattro (patisiran) is approved for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis. The injection recorded sales of $91.5 million in the second quarter, down 40% on a reported basis. Onpattro sales fell short of the Zacks Consensus Estimate of $94 million as well as our estimate of $98.6 million.
In June 2022, the FDA approved Alnylam’s RNAi therapeutic, Amvuttra, for the treatment of adult patients with polyneuropathy of hATTR amyloidosis. The European Commission approved Amvuttra for treating hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy in September.
Amvuttra generated sales worth $132.1 million in the second quarter. The initial uptake for the product has been encouraging with new patients starting treatment as well as several patients switching from Onpattro. Amvuttra sales beat the Zacks Consensus Estimate of $123 million as well as our estimate of $110.2 million.
As of Jun 30, 2023, Alnylam attained more than 3,490 hATTR amyloidosis patients with polyneuropathy worldwide on commercial treatment with Onpattro or Amvuttra, representing 10% quarterly patient growth. Givlaari (givosiran), approved for the treatment of acute hepatic porphyria, recorded sales of $57.9 million, reflecting a year-over-year increase of 28% on both reported basis and at CER. Givlaari sales surpassed the Zacks Consensus Estimate of $52 million as well as our estimate of $52.9 million.
Oxlumo (lumasiran) recorded global net product revenues of about $24.2 million in the second quarter of 2023, reflecting a year-over-year increase of 62% on both reported basis and at CER. Oxlumo sales also fell short of the Zacks Consensus Estimate of $27.3 million and missed our estimate of $25.8 million.
Adjusted research and development (R&D) expenses increased 11% year over year to $215.7 million, driven by an increase in headcount to support our R&D pipeline along with higher expenses incurred in development and study activities.
Adjusted selling, general and administrative (SG&A) expenses rose 14% year over year to $171.7 million because of higher headcount expenses and supporting the launch activities for Amvuttra.
Cash, cash equivalents and marketable securities as of Jun 30, 2023, amounted to $2.1 billion, which remained unchanged from the last quarter’s reported figure.
2023 Financial Guidance Reaffirmed
Alnylam maintained its expectations for net product revenues for Onpattro, Amvuttra, Givlaari and Oxlumo in the range of $1,200-$1,285 million for 2023. Our estimate for net product revenues is pegged at $1,216.6 million for 2023.
Net revenues from collaborations and royalties are expected in the range of $100-$175 million (no change). Adjusted R&D and SG&A expenses are anticipated in the band of $1,575-$1,650 million (no change).
The Zacks Consensus Estimate for total revenues in 2023 is pegged at $1.43 billion.
Pipeline Updates
In May 2023, Alnylam announced new positive results from an interim analysis of exploratory data from the open-label extension (OLE) period of the phase III APOLLO-B study of patisiran for the treatment of ATTR amyloidosis with cardiomyopathy.
The 18-month data, from the interim analysis of the OLE period, was submitted to the FDA as an amendment to the supplemental new drug application for patisiran for the treatment of the cardiomyopathy of ATTR amyloidosis. A decision from the regulatory body is expected on Oct 8, 2023.
Recently, Alnylam announced entering into a strategic collaboration with Roche RHHBY to co-develop and co-commercialize zilebesiran, a subcutaneously administered RNAi therapeutic targeting angiotensinogen, for the treatment of hypertension.
Per ALNY, Roche is a well-suited partner for the development of zilebesiran with its expertise in developing and commercializing novel therapies in complex markets. The agreement will also provide Alnylam access to Roche’s abundant resources and global infrastructure.
Alnylam Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
Alnylam Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Alnylam Pharmaceuticals, Inc. Quote
Other Updates
In a separate press release, Agios Pharmaceuticals AGIO announced entering into a worldwide license agreement with Alnylam to acquire the exclusive rights to develop and commercialize Alnylam’s novel preclinical siRNA targeting TMPRSS6, as a potential disease-modifying treatment for patients with polycythemia vera. The agreement brings Agios’ deep scientific expertise and capabilities in rare hematologic diseases, together with Alnylam’s world-class siRNA platform.
Per the terms of the agreement, Agios is liable to make an upfront payment of $17.5 million to Alnylam for an exclusive global license to the TMPRSS6 siRNA program. Furthermore, ALNY is entitled to receive up to $130 million in potential development and regulatory milestone payments on top of sales-based milestone and tiered royalty payments.
The agreement also states that Agios is set to assume sole responsibility for all development, regulatory and commercial activities and costs related to the program, after receiving initial manufacturing support from Alnylam for phase I.
Zacks Rank
Alnylam currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Agios Pharmaceuticals, Inc. (AGIO) : Free Stock Analysis Report
