Alnylam (ALNY) Q4 Loss Narrower Than Expected, Stock Dips 10%
Alnylam Pharmaceuticals ALNY reported a loss of $1.10 per share in the fourth quarter of 2023, narrower than the Zacks Consensus Estimate of a loss of $1.20. The company had incurred a loss of $1.68 per share in the year-ago quarter.
The fourth-quarter loss included stock-based compensation expenses, unrealized gain on equity securities and loss on extinguishment of debt. Excluding these items, the adjusted loss was 77 cents per share, narrower than the adjusted loss of $1.39 reported in the year-ago quarter.
Alnylam recorded total revenues of $440 million in the quarter, which missed the Zacks Consensus Estimate of $446 million. In the year-ago quarter, total revenues were $335 million. Net product revenues were $346 million in the reported quarter, up 32% year over year on a reported basis and 30% at constant exchange rate (CER), driven by increased patient numbers for Onpattro (patisiran), Givlaari (givosiran), Oxlumo (lumasiran) and the newly approved Amvuttra (vutrisiran).
Net revenues from collaborators were $76 million, up 8% from $71 million reported in the year-ago quarter, primarily driven by revenues recognized under the company’s licensing agreement with Roche RHHBY and its collaboration agreement with Novartis NVS regarding the achievement of specified commercialization and regulatory milestones.
We would like to remind the investors that Alnylam announced that it entered a strategic collaboration with Roche to co-develop and co-commercialize zilebesiran for the treatment of hypertension in July 2023. The upfront payment amount received from RHHBY, together with several milestone payments, amounts to a potential deal value of up to $2.8 billion.
Alnylam has granted Novartis exclusive and worldwide rights to manufacture and commercialize RNAi therapeutics targeting PCSK9 for the treatment of hypercholesterolemia and other human diseases, including Leqvio (inclisiran), under the ongoing collaboration.
Novartis has also received FDA approval to expand Leqvio’s label to include earlier use in patients with elevated LDL-C who have an increased risk of heart disease as an adjunct to diet and statin therapy.
ALNY also earns revenues under its ongoing collaboration agreement with Regeneron REGN.
Alnylam, in partnership with Regeneron, is developing ALN-APP for treating early-onset Alzheimer’s disease.The company, in collaboration with REGN, is also advancing cemdisiran, an investigational RNAi therapeutic for the treatment of complement-mediated diseases.
ALNY is entitled to receive license fees from these collaboration agreements.
In the reported quarter, the company recorded royalty revenues of $17 million. In the year-ago quarter, it recorded royalty revenues of $2.7 million.
However, the company’s stock lost 10.2% in the last trading session due to the mixed fourth-quarter results. The decline in the stock price also signifies investors’ skepticism regarding the changes to the heart disease study’s plan. Shares of Alnylam have plunged 33.8% in the past year compared with the industry’s decline of 11.5%.
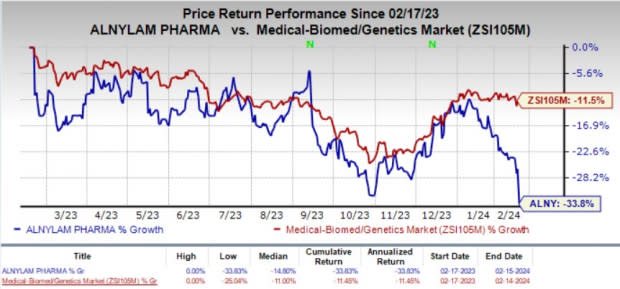
Image Source: Zacks Investment Research
Quarter in Detail
Onpattro (patisiran) is approved for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis. The injection recorded sales of $79 million in the fourth quarter, down 35% on a reported basis. Onpattro sales beat the Zacks Consensus Estimate of $75 million and matched our model estimate.
In June 2022, the FDA approved Alnylam’s RNAi therapeutic, Amvuttra, for the treatment of adult patients with polyneuropathy of hATTR amyloidosis. The European Commission approved Amvuttra for treating hATTR amyloidosis in adult patients with stage 1 or 2 polyneuropathy in September.
Amvuttra generated sales worth $175 million in the reported quarter, up 156% on a reported basis. The initial uptake for the product has been encouraging, with new patients starting treatment as well as several patients switching from Onpattro. Amvuttra sales marginally beat the Zacks Consensus Estimate of $174 million and matched our model estimate.
As of Dec 31, 2023, more than 4,060 patients were on commercial Onpattro or Amvuttra treatment worldwide, up from 3,790 patients at the end of the third quarter, representing 7% quarterly patient growth.
Givlaari (givosiran), approved for the treatment of acute hepatic porphyria, recorded sales of $59 million, reflecting a year-over-year increase of 26% on a reported basis. Givlaari sales matched both the Zacks Consensus Estimate as well as our estimate.
Oxlumo (lumasiran) recorded global net product revenues of about $32.7 million in the fourth quarter of 2023, reflecting a year-over-year increase of 37% on a reported basis. However, Oxlumo sales slightly missed the Zacks Consensus Estimate of $33 million and our estimate of $32.8 million.
Adjusted research and development (R&D) expenses increased 3% year over year to $253 million, driven by a rise in headcount to support the company’s R&D pipeline, along with higher expenses incurred in development and study activities.
Adjusted selling, general and administrative (SG&A) expenses declined 5% year over year to $175 million due to a decrease in legal expenses as Alnylam’s lawsuit for patent infringement closed in August 2023.
Cash, cash equivalents and marketable securities as of Dec 31, 2023, amounted to $2.4 billion, the same as observed on Sep 30, 2023.
2023 Results
For 2023, Alnylam generated total revenues of $1.83 billion compared with $1.04 billion recorded in 2022. The figure matched the Zacks Consensus Estimate.
For the year, the company reported a loss of $3.52 per share, narrower than a loss of $9.30 posted in 2022 due to higher revenues. The reported figure was, however, wider than the Zacks Consensus Estimate of a loss of $3.47 per share.
Alnylam Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
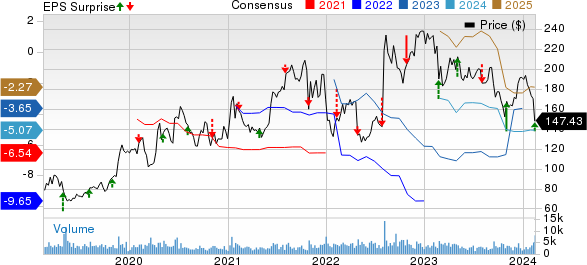
Alnylam Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Alnylam Pharmaceuticals, Inc. Quote
2024 Financial Guidance
Alnylam expects net product revenues for Onpattro, Amvuttra, Givlaari and Oxlumo in the range of $1.4-$1.5 billion for 2024.
Net revenues from collaborations and royalties are expected in the range of $325-$425 million. Adjusted R&D and SG&A expenses are anticipated in the band of $1.675-$1.775 billion.
Pipeline Updates
In the same earnings release, Alnylam announced having updated its statistical analysis plan for the phase III study of vutrisiran in patients with ATTR amyloidosis with cardiomyopathy. It includes updates to the primary and secondary endpoint structure, as well as study exposure. Top-line results are expected to be reported around mid-2024.
Zacks Rank
Alnylam currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
