Alnylam (ALNY) Receives CRL for the Label Expansion of Onpattro
Alnylam Pharmaceuticals, Inc. ALNY announced that the FDA has issued a Complete Response Letter (CRL) in response to the company’s supplemental New Drug Application (sNDA) for the label expansion of Onpattro (patisiran).
The sNDA is seeking regulatory approval for Onpattro to treat the cardiomyopathy of transthyretin-mediated (ATTR) amyloidosis.
Per ALNY, the CRL was issued to the company on the grounds that the existing study data did not establish the clinical meaningfulness of Onpattro’s treatment effects for the cardiomyopathy of ATTR amyloidosis. Hence, the sNDA could not be approved in its present form.
The sNDA application was based on the positive results from the APOLLO-B study of patisiran to treat the cardiomyopathy of ATTR amyloidosis. The study observed the favorable effects of treatment with patisiran on functional capacity, health status and quality of life in patients with ATTR amyloidosis with cardiomyopathy against placebo.
However, Alnylam clarified that the CRL did not identify any issues with respect to clinical safety, study conduct, drug quality or the manufacturing of Onpattro.
The stock lost 4.9% on Monday, indicating investors’ disappointment regarding the regulatory setback. Year to date, shares of Alnylam have declined 29.2% compared with the industry’s 18.3% fall.
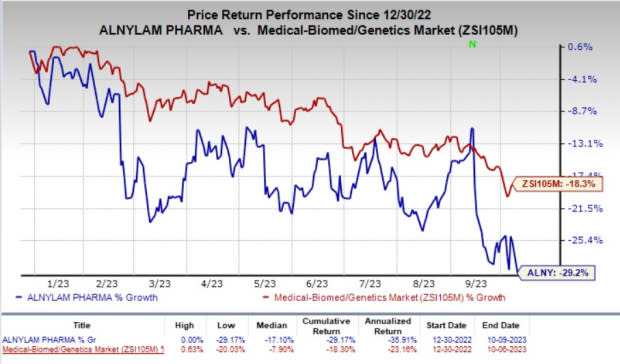
Image Source: Zacks Investment Research
Patisiran is currently marketed as Onpattro in the United States and Europe for the treatment of the polyneuropathy of hereditary ATTR amyloidosis in adults. Onpattro is the first and only FDA-approved treatment for this indication.
ALNY has further clarified that the CRL does not pertain to, nor impact, the commercial availability of Onpattro in its approved indication.
Based on the FDA’s CRL, Alnylam has decided to discontinue pursuing an expanded indication for Onpattro in the United States. However, the company will continue to focus on the phase III HELIOS-B label-expanding study of Amvuttra (vutrisiran) in the treatment of the cardiomyopathy of ATTR amyloidosis, expecting top-line data in early 2024.
Amvuttra, an RNAi therapeutic drug, received FDA approval in June 2022, for the treatment of adult patients with the polyneuropathy of hereditary ATTR (hATTR) amyloidosis. Amvuttra is also approved in the EU for the treatment of hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy.
Alnylam also remains focused on developing its new investigational candidate, ALN-TTRsc04, which utilizes the company’s IKARIA technology. Management believes that ALN-TTRsc04 has the potential for greater than 90% TTR knockdown with once annual dosing.
It is to be noted that the CRL was issued by the FDA despite a positive opinion from the FDA’s advisory committee regarding the label expansion of Onpattro to treat the cardiomyopathy of ATTR amyloidosis, which was announced last month.
Per Alnylam, out of 12 expert panelists in the committee, nine voted in favor of the company, indicating that the benefits of Onpattro outweigh its risks for the treatment of the cardiomyopathy of ATTR amyloidosis.
Alnylam Pharmaceuticals, Inc. Price and Consensus
Alnylam Pharmaceuticals, Inc. price-consensus-chart | Alnylam Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Alnylam currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the overall medical sector are Corcept Therapeutics CORT, Annovis Bio ANVS and Better Therapeutics BTTX, each carrying a Zacks Rank #2 (Buy) at present.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has remained constant at 78 cents. During the same period, the estimate for Corcept’s 2024 earnings per share has also remained constant at 83 cents. Year to date, shares of CORT have gained 31.2%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2023 loss per share has remained constant at $4.38. During the same period, the estimate for Annovis’ 2024 loss per share has also remained constant at $2.77. Year to date, shares of ANVS have lost 40.1%.
ANVS’ earnings beat estimates in three of the trailing four quarters and missed the mark on one occasion, delivering an average surprise of 13.40%.
In the past 30 days, the Zacks Consensus Estimate for Better Therapeutics’ 2023 loss per share has remained constant at 98 cents. During the same period, Better Therapeutics’ 2024 loss per share has also remained constant at 80 cents. Year to date, shares of BTTX have lost 64.7%.
BTTX’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 24.22%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
Better Therapeutics, Inc. (BTTX) : Free Stock Analysis Report
