Alvotech (ALVO) Up on BLA Update for Two Biosimilar Candidates
Alvotech ALVO announced that the FDA has completed the reinspection of the company’s Iceland facility that started earlier this month. Alvotech received a form 483 with one observation after the regulatory body’s reinspection.
Previously, certain deficiencies were found at the Iceland facility.
Based on this, Alvotech remains confident about receiving approval for its two biosimilar candidates, AVT02 and AVT04, in the United States by Feb 24, 2024 and Apr 16, 2024, respectively.
Shares of the company were up 15.3% on Jan 19 following the announcement of the news.
AVT02 is a high-concentration biosimilar of AbbVie’s Humira (adalimumab), a drug used to treat autoimmune disorders such as rheumatoid arthritis and psoriasis.
AVT04 is a biosimilar candidate of Johnson & Johnson’s JNJ blockbuster immunology drug, Stelara (ustekinumab).
Shares of Alvotech have rallied 18.5% in the past year against the industry’s decline of 15.2%.
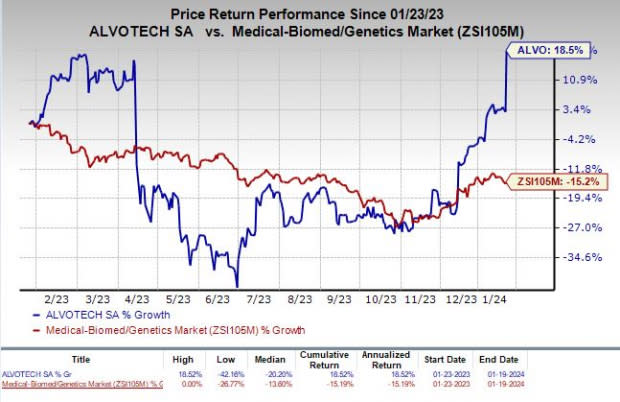
Image Source: Zacks Investment Research
Per the company, the FDA had previously accepted the resubmitted biologics license application (BLA) for AVT02 and set a Biosimilar User Fee Act (BsUFA) goal date of Feb 24, 2024. Similarly, it had accepted the resubmitted BLA for AVT04 and set a BsUFA goal date of Apr 16, 2024.
The satisfactory outcome of Alvotech’s facility reinspection by the FDA held the key to the approval of the BLAs for AVT02 and AVT04.
Per a settlement agreement with JNJ, the license entry date for AVT04 was set as Feb 21, 2025 in the United States. However, a potential nod now to the resubmitted BLA for AVT04 could enable the early entry of the biosimilar version of Stelara in the United States.
J&J expected Stelara biosimilars to be launched in 2025.
Earlier this month, the European Commission granted a marketing authorization to AVT04 under the brand name of Uzpruvo. The approval marked the first biosimilar version of Stelara to be granted marketing authorization in Europe.
The approval in Europe was expected as in November 2023, the European Medicines Agency’s Committee for Medicinal Products for Human Use rendered a positive opinion for approving Uzpruvo for treating Crohn’s disease, psoriasis and psoriatic arthritis.
Zacks Rank & Stocks to Consider
Alvotech currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are CytomX Therapeutics, Inc. CTMX and Puma Biotechnology, Inc. PBYI, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for CytomX Therapeutics’ 2024 loss per share have narrowed from 22 cents to 6 cents. In the past year, shares of CTMX have plunged 40.3%.
CytomX Therapeutics beat estimates in three of the last four quarters while missing the same on the remaining occasion. CTMX delivered a four-quarter earnings surprise of 45.44%, on average.
In the past 60 days, estimates for Puma Biotechnology’s 2024 earnings per share have improved from 62 cents to 69 cents. In the past year, shares of PBYI have risen 5.8%.
Earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
CytomX Therapeutics, Inc. (CTMX) : Free Stock Analysis Report
Alvotech (ALVO) : Free Stock Analysis Report
