Alzheimer's Disease Space Evolves in 2023: Stocks in Focus
The Alzheimer's disease (AD) space continued to be in the spotlight in 2023 with the approval of Biogen BIIB and Eisai’s drug Leqembi, which was initially granted accelerated approval in January.
In July, the drug was granted traditional approval based on data from Eisai’s late-stage global Clarity AD clinical study, wherein Leqembi met its primary endpoint and all key secondary endpoints with statistically significant results.
The study confirmed the clinical benefit of Leqembi. The approval made Leqembi the first and only approved anti-amyloid Alzheimer's disease treatment shown to reduce the rate of disease progression and slow down cognitive impairment in the early and mild dementia stages of the disease.
Alzheimer's is a progressive, fatal disease of the brain characterized by a decline in memory, language and other thinking skills, as well as changes in mood and behavior.
We remind readers that this is the second drug from Biogen and Eisai that has won FDA approval. The regulatory body had earlier approved their controversial AD drug, Aduhelm. However, the euphoria surrounding Aduhelm faded soon as the drug witnessed a slow launch due to reimbursement issues as its efficacy was scrutinized.
Nevertheless, investors are now optimistic about the pipeline candidates of other companies developing treatments for AD.
Companies like Eli Lilly and Prothena PRTA continue to be in focus in this regard. The space is evolving as there is a renewed interest in AD vaccines now.
The interest comes on the heels of data showing an increase of amyloid-related imaging abnormalities (ARIA) observed in the case of the approved therapies, reflected as vasogenic edema or sulcal effusion (ARIA-E) or as hemosiderin deposits such as microhemorrhages and superficial siderosis (ARIA-H). The ease of administration and less frequent dosing (Leqembi requires twice-monthly infusions) associated with vaccines are also added benefits, which have propelled a few companies to evaluate AD vaccines.
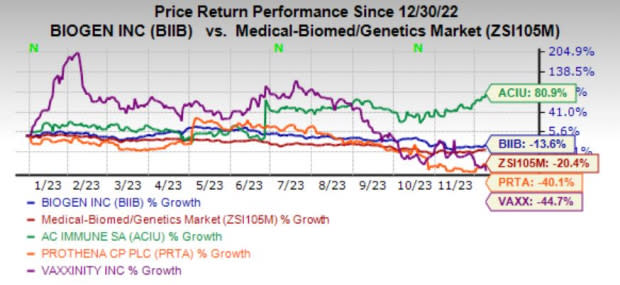
Image Source: Zacks Investment Research
Earlier in the year, the FDA granted Fast Track designation to AC Immune’s ACIU anti-amyloid beta active immunotherapy (vaccine)-candidate, ACI-24.060, for the treatment of AD.
Per AC Immune, by inducing a polyclonal response, including antibodies against both oligomeric amyloid beta and pyroglutamate- amyloid beta, ACI-24.060 targets the same toxic species as disease-modifying anti-amyloid beta monoclonal antibodies that slowed AD progression in phase III studies.
The company plans to release more data in the first half of 2024 showing the effect of ACI-24.060 on amyloid plaque reduction, a surrogate marker for disease modification.
AC Immune is also developing a potential first-in-class anti-phosphorylated-Tau vaccine candidate for AD with Janssen Pharmaceuticals.
Vaxxinity, Inc. VAXX also obtained Fast Track Designation for its AD vaccine UB-311 in the United States. UB-311 targets toxic forms of aggregated amyloid beta in the brain to fight AD.
In August 2023, Vaxxinity announced publication data from a phase IIa study that showed that UB-311 “was safe and well-tolerated,” with early clinical data demonstrating a trend for slowing cognitive decline in mild AD.
The phase IIa data showed the safety, tolerability, immunogenicity, and early clinical efficacy of UB-311 when evaluated with quarterly or biannual booster doses. UB-311 demonstrated robust, rapid and titrated antibody response to amyloid beta. It was generally well-tolerated, with no cases of ARIA-E and limited cases of asymptomatic ARIA-H.
Prothena has a portfolio of programs for the potential treatment of AD, including PRX012, which targets amyloid beta and PRX123, a novel dual amyloid beta-tau (Aβ-Tau) vaccine.
Prothena’s PRX012, too, is a next-generation subcutaneous antibody being developed for the treatment of AD. It targets a key epitope at the N-terminus of Aβ with high binding potency. Two preclinical studies had earlier shown superior binding characteristics of PRX012, demonstrating a 20-fold higher affinity to Aβ soluble protofibrils when compared with Leqembi and cleared pyroglutamate-modified Aβ at lower concentrations when compared with Eli Lilly’s investigational candidate donanemab.
Prothena is also developing a dual Aβ-Tau vaccine, PRX123, — a potential prevention and treatment for AD — to target key epitopes within Aβ and tau proteins to promote amyloid clearance and block pathogenic tau interaction. An investigational new drug application filing for the vaccine is anticipated by the end of 2023.
Reportedly, attempts were made earlier on as well to develop AD vaccines but were shelved after enrolled participants developed a brain inflammation known as meningoencephalitis.
Nevertheless, the approval of Leqembi has now shown how AD treatments work. Hence, AD vaccine makers are now better equipped to develop shots that will not only generate response but also keep inflammation in control.
These experimental vaccines still have a long and challenging path ahead. Breakthroughs in this space will be instrumental in driving growth.
The abovementioned stocks carry a Zacks Rank #3 (Hold) each. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Prothena Corporation plc (PRTA) : Free Stock Analysis Report
AC Immune (ACIU) : Free Stock Analysis Report
Vaxxinity, Inc. (VAXX) : Free Stock Analysis Report
