Amgen's (AMGN) Blincyto sBLA Gets FDA Nod in Treating Leukemia
Amgen AMGN announced that the FDA has approved its supplemental biologics license application (sBLA) for Blincyto (blinatumomab) for the treatment of adults and pediatric patients with CD19-positive B-cell precursor acute lymphoblastic leukemia (B-ALL). Following the approval of the sBLA, Blincyto can be used to treat patients experiencing first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1%.
Per Amgen, the approval of Blincyto, a bispecific T-cell engager (BiTE) immuno-oncology therapy, is based on positiveadditional data from two phase III studies that were submitted to the regulatory body for review. AMGN previously received accelerated approval for Blincyto in the same indication in March 2018, which is now converted to full approval.
Year to date, shares of Amgen have lost 13.5% compared with the industry’s 7.6% decline.
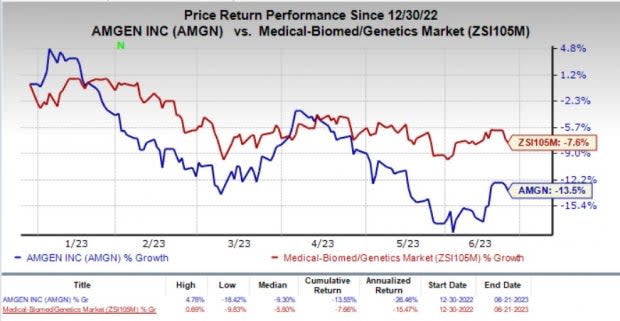
Image Source: Zacks Investment Research
MRD is a condition when the presence of cancer cells remains detectable, despite a patient having achieved complete remission by conventional assessment. The detection of such cancer cells is only possible through highly sensitive instruments.
The 2018 accelerated approval of Blincyto was the first-and-only approved BiTE immunotherapy as well as the first-and-only therapy to be FDA-approved for MRD.
Management claimed that in a phase II study, approximately 80% of adult patients treated with Blincyto were observed to have experienced a complete MRD response. The full approval reportedly reinforces Blincyto as the standard of care for patients with MRD at baseline after remission.
We would like to remind the investors that Blincyto was previously granted breakthrough therapy and priority review designations by the FDA. Blincyto is now approved for relapsed or refractory CD19-positive B-cell precursor ALL in adults and pediatric patients in the United States.
In EU, Blincyto is also approved as a monotherapy in the treatment of several leukemia indications.
Amgen is committed to developing Blincyto for additional indications, including MRD-negative B-ALL, studies designed to minimize chemotherapy as well as investigating a subcutaneous formulation of the same, in the quest of addressing remaining unmet needs for patients.
Amgen is also investigating BiTE immuno-oncology therapies, given its potential to treat a wide variety of cancers.
Amgen Inc. Price and Consensus
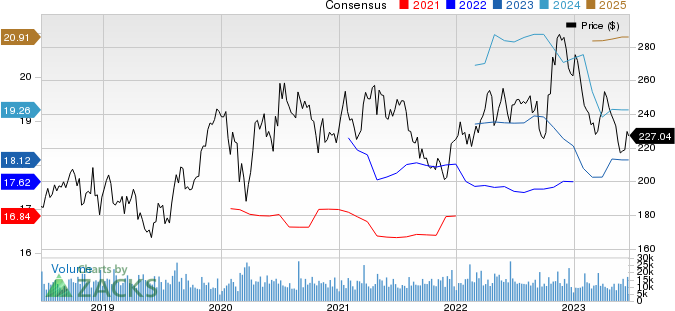
Amgen Inc. price-consensus-chart | Amgen Inc. Quote
Zacks Rank and Stocks to Consider
Amgen currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Adaptimmune Therapeutics ADAP, Akero Therapeutics AKRO and ADMA Biologics, Inc. ADMA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Adaptimmune Therapeutics’ 2023 loss per share has narrowed from 63 cents to 46 cents. During the same period, the estimate for Adaptimmune Therapeutics’ 2024 loss per share has narrowed from 59 cents to 56 cents. Year to date, shares of ADAP have fallen by 30.9%.
ADAP beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 36.89%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $2.96 to $2.80. During the same period, the estimate for Akero Therapeutics’ 2024 loss per share has narrowed from $3.40 to $3.27. Year to date, shares of AKRO have gained 3.8%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
In the past 90 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 19 cents to 9 cents. The consensus estimate for 2024 earnings is currently pegged at 7 cents per share. Year to date, shares of ADMA have gained by 1.8%.
ADMA beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 19.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Amgen Inc. (AMGN) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Adaptimmune Therapeutics PLC (ADAP) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
