Amylyx (AMLX) Up 51% After FDA Committee Endorses ALS Drug
Shares of Amylyx Pharmaceuticals AMLX surged 51% on Sep 8 after management announced that an FDA Advisory Committee has recommended approving the company’s new drug application (“NDA”) for AMX0035, an oral medicine to treat amyotrophic lateral sclerosis (“ALS”).
The FDA’s Peripheral and Central Nervous System Drugs Advisory Committee (“PCNSDAC”) voted 7:2, with the majority favoring the drug’s approval for ALS. A final decision from the FDA is expected by Sep 29, 2022. Note that the FDA is not bound to follow the advice of its advisory committees
Shares of Amylyx were mostly likely up as investors anticipate a potential approval of the NDA later this month. Generally, the FDA’s decision stands in alignment with its advisory committees.
In the year so far, the stock has surged 49.6% against the industry’s 23.4% fall.
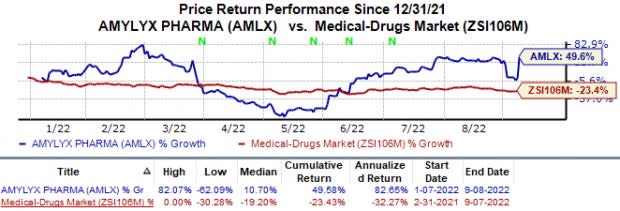
Image Source: Zacks Investment Research
A similar regulatory application has also been submitted to the European Medicines Agency (EMA) and a decision is expected by next year.
This positive decision from the PCNSDAC is based on data from the phase II CENTAUR study, which evaluated the drug in ALS compared with placebo in ALS patients. The study achieved its primary efficacy endpoint as participants administered AMX0035 demonstrated a significant slow-down of ALS progression and functional decline. Treatment with the drug also increased overall survival compared with patients who were administered placebo.
AMX0035 has already received marketing authorization in Canada and is being marketed under the trade name Albrioza. This authorization was received by Amylyx earlier this year in June. The drug is yet to be approved in Europe or the United States.
This is the second PCNSDAC meeting convened to review the NDA for AMX0035 in ALS. The first meeting was convened earlier this year in March, wherein the committee members voted against approving the drug based on data submitted by the company at the time. After the company submitted additional data analyses from clinical studies on AMX0035, the FDA deemed such data as major amendments to the NDA and decided to reconvene a second PCNSDAC meeting. This also led to extending the target action date from June to September end. The NDA was also granted Priority Review Designation by the FDA last year in December.
Apart from ALS, AMX0035 is also being evaluated for Alzheimer’s disease. Amylyx also plans to submit an investigational new drug (IND) application to the FDA later this year to evaluate the drug as a treatment for Wolfman’s Syndrome.
Amylyx Pharmaceuticals, Inc. Price
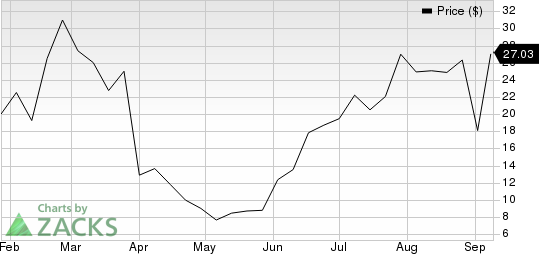
Amylyx Pharmaceuticals, Inc. price | Amylyx Pharmaceuticals, Inc. Quote
Zacks Rank & Stocks to Consider
Amylyx currently carries a Zacks Rank #3 (Hold).
Some better-ranked stock in the overall healthcare sector includes Kamada KMDA, Morphic MORF and Sesen Bio SESN, each of which has a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Kamada’s 2022 earnings per share have risen from 1 cent to 26 cents. Shares of Kamada have lost 25.2% in the year-to-date period.
Earnings of Kamada missed estimates in three of the last four quarters and beat the mark just once, witnessing a negative surprise of 212.50%, on average. In the last reported quarter, KMDA’s earnings beat estimates by 450%.
In the past 60 days, estimates for Morphic’s 2022 loss per share have narrowed from $3.47 to $1.75. Loss estimates for 2023 have narrowed from $3.96 to $3.77 during the same period. Shares of Morphic have lost 36.6% in the year-to-date period.
Earnings of Morphic beat estimates in three of the last four quarters and missed the mark just once, witnessing a surprise of 48.29%, on average. In the last reported quarter, MORF delivered an earnings surprise of 183.95%.
Estimates for Sesen Bio’s 2023 bottom line have narrowed from 27 cents to 1 cent in the past 60 days. Share prices of Sesen Bio have fallen 20.1% in the year-to-date period.
Earnings of Sesen Bio beat estimates in each of the last four quarters, the average surprise being 89.49%. In the last reported quarter, Sesen Bio delivered an earnings surprise of 61.54%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Kamada Ltd. (KMDA) : Free Stock Analysis Report
Amylyx Pharmaceuticals, Inc. (AMLX) : Free Stock Analysis Report
SESEN BIO, INC. (SESN) : Free Stock Analysis Report
Morphic Holding, Inc. (MORF) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
