Anavex (AVXL) Falls 35% on Mixed Rett Syndrome Study Results
Anavex Life Sciences Corp. AVXL reported mixed results from the mid to late-stage EXCELLENCE study evaluating the clinical efficacy, safety and tolerability of its investigational candidate, 30 mg ANAVEX2-73(blarcamesine), in pediatric patients (aged 5-17 years) suffering from Rett Syndrome (RTT).
The company’s shares lost 35% in the last trading session as the mixed results from the study did not impress the investors.
The phase II/III EXCELLENCE study enrolled 92 pediatric patients with RTT, who were randomized to receive either ANAVEX2-73 or placebo in the ratio 2:1 for 12 weeks, followed by a week 16 safety visit. Per the data readout, the study showed improvement on the key co-primary endpoint Rett Syndrome Behavior Questionnaire (RSBQ) after 12 weeks. However, the other co-primary endpoint of the Clinical Global Impression – Improvement scale (CGI-I) was not met.
Anavex also reported results from an ad-hoc analysis of the EXCELLENCE study data. Per the analysis results, although patients treated with ANAVEX2-73 demonstrated improved LS Mean points on their RSBQ total score compared with LS Mean in placebo-treated patients, the LS Mean difference between the two groups did not reach statistical significance, at week 12.
However, following the same metric, patients treated with ANAVEX2-73 demonstrated a rapid onset of action with improvements at week fourcompared with placebo-treated patients and the difference in efficacy achieved statistical significance.
Over the past year, shares of AVXL have lost 39.4% compared with the industry’s 11.9% decline.
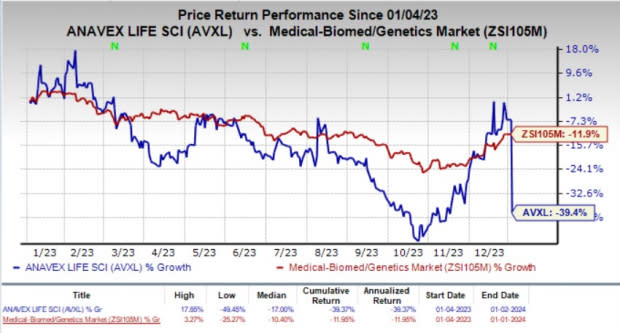
Image Source: Zacks Investment Research
Anavex stated that ANAVEX2-73 compares favorably with other placebo-controlled RTT studies, in terms of absolute RSBQ improvements. The company further reported that the key secondary endpoint of the study trended in favor of treatment with the candidate. Collectively, treatment with ANAVEX2-73 had a positive impact, demonstrating improvements in multiple areas like repetitive movements, nighttime disruptive behaviors and social avoidance.
In the press release, Anavex stated that it observed a large placebo effect, which could have masked the therapeutic effect of the investigational candidate, thus failing to meet statistical significance in several key metrics. The company believes to have identified the probable causes.
Although data analysis is currently ongoing, management’s early conclusion claims that the placebo rate could have been higher in the study due to a slight imbalance in disease severity at baseline, across the treatment arms and in the randomization ratio.
Anavex intends to further analyze the collective results and discuss the same with the regulatory authorities to determine the next steps.
A preliminary review of the safety data from the EXCELLENCE study regarding treatment with ANAVEX2-73 did not indicate any new safety signals. The candidate demonstrated a favorable and manageable safety profile. Treatment-related adverse events were mostly mild to moderate in severity.
Anavex’s 48-week open-label extension study of ANAVEX2-73 in RTT is also currently ongoing. Positive real-world evidence feedback regarding improvement in the quality of life of pediatric RTT patients upon continued treatment with ANAVEX2-73 under the company’s Compassionate Use Program is reassuring.
ANAVEX2-73 currently enjoys the FDA’s Fast Track, Rare Pediatric Disease and Orphan Drug designation in the United States for the treatment of RTT.
We would like to remind the investors that the company recently received an agreement from the Committee for Medicinal Products for Human Use within the EU regulatory body for the submission of a marketing authorization-seeking application of oral blarcamesine for Alzheimer’s disease.
Acadia Pharmaceuticals ACAD currently markets the first and only drug approved by the FDA for the treatment of RTT in the United States. Acadia’s Daybue (trofinetide) received FDA approval in March 2023 for the treatment of RTT in adult and pediatric patients aged two years and older. Following the approval, the drug was launched in the U.S. market in mid-April 2023.
Acadia in-licensed exclusive rights to develop and commercialize trofinetide in North America from Neuren in 2018. However, Neuren had retained the rights to develop and commercialize trofinetide for all indications outside North America. However, following a deal to expand the current licensing agreement with Neuren in July 2023, ACAD acquired rights to market trofinetide outside North America, along with exclusive global rights to Neuren’s development candidate, NNZ-2591, in RTT and Fragile X syndrome.
Anavex Life Sciences Corp. Price and Consensus
Anavex Life Sciences Corp. price-consensus-chart | Anavex Life Sciences Corp. Quote
Zacks Rank and Other Stocks to Consider
Anavex currently carries a Zacks Rank #2 (Buy).
Some other top-ranked drug/biotech stocks worth mentioning are Puma Biotechnology, Inc. PBYI and ADMA Biologics ADMA. While PBYI sports a Zacks Rank #1 (Strong Buy), ADMA carries a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has increased from 72 cents to 73 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has increased from 64 cents to 69 cents. Over the past year, shares of PBYI have risen 7.7%.
PBYI’s earnings beat estimates in three of the last four quarters and missed the same once, delivering an average surprise of 76.55%.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 3 cents to 2 cents. The consensus estimate for ADMA Biologics’ 2024 EPS is pegged at 18 cents. Over the past year, shares of ADMA have risen 24.7%.
ADMA's earnings beat estimates in three of the trailing four quarters and met the same in one, delivering an average surprise of 63.57%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
Anavex Life Sciences Corp. (AVXL) : Free Stock Analysis Report
