Anavex (AVXL) Rises as CHMP Deems Blarcamesine Eligible for MAA
Anavex Life Sciences Corp. AVXL announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) considers its pipeline candidate, oral blarcamesine, eligible for a centralized regulatory review in the EU.
This indicates that Anavex may file a single marketing application to the European Medicines Agency. The company initiated the regulatory submission of blarcamesine for the treatment of Alzheimer’s disease to the EMA in November 2023.
The marketing authorization application, upon potential approval, will grant the company direct access to the European Union market. Anavex is planning to submit a marketing authorization application for blarcamesine at the earliest possible date in 2024.
Shares of the Anavex were up 13.2% on Dec 19 following the announcement of the news. The stock has rallied 15.6% in the past year against the industry’s decline of 19.6%.
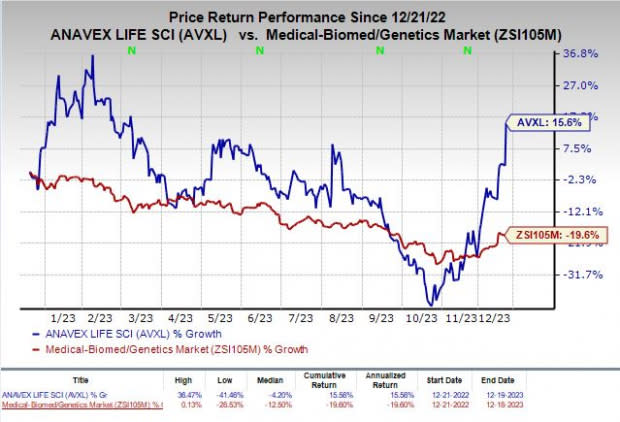
Image Source: Zacks Investment Research
Per the company, blarcamesine is being evaluated in a IIb/III clinical study for Alzheimer's disease. Besides showing significant improvement in dementia symptoms in the study, treatment with blarcamesine also led to a reduction in pathological aggregation of amyloid in early Alzheimer’s disease and reduced brain volume loss, a renowned marker of neurodegeneration.
Anavex’s orally administered blarcamesine is an easier-to-use formulation. The oral treatment does not require complex logistics resources and added personnel for drug administration and monitoring for brain edema and brain bleeds.
Anavex is also evaluating blarcamesine in several other central nervous system indications. The candidate is being developed in mid-to-late-stage studies for treating Rett syndrome, Parkinson's disease, schizophrenia, Fragile X and other rare diseases.
Zacks Rank & Other Stocks to Consider
Anavex currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the healthcare sector are Journey Medical Corporation DERM, Entrada Therapeutics, Inc. TRDA and Puma Biotechnology, Inc. PBYI, each sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Journey Medical’s 2023 loss per share have narrowed from $1.28 to 16 cents. Meanwhile, loss per share estimates for 2024 have narrowed from 41 cents to 35 cents. In the past year, shares of DERM have surged 568.4%.
Earnings of Journey Medical beat estimates in one of the last four quarters while missing the same on the remaining three occasions. DERM delivered a four-quarter earnings surprise of 118.25%, on average.
In the past 60 days, estimates for Entrada Therapeutics’ 2023 loss per share have narrowed from $2.07 to 9 cents. Meanwhile, loss per share estimates for 2024 have narrowed from $2.35 to $2.04. In the past year, shares of TRDA have decreased 11.7%.
Earnings of Entrada Therapeutics beat estimates in three of the last four quarters while missing the same on the remaining occasion. TRDA delivered a four-quarter average earnings surprise of 70.68%.
In the past 60 days, estimates for Puma Biotechnology’s 2023 earnings per share have improved from 67 cents to 72 cents. During the same period, earnings per share estimates for 2024 have moved up from 55 cents to 64 cents. In the past year, shares of PBYI have lost 19.8%.
Earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Journey Medical Corporation (DERM) : Free Stock Analysis Report
Anavex Life Sciences Corp. (AVXL) : Free Stock Analysis Report
Entrada Therapeutics, Inc. (TRDA) : Free Stock Analysis Report
