Apellis (APLS) Dips on Layoffs Amid Syfovre Safety Issues
Apellis Pharmaceuticals APLS announced its plans to start acomprehensive corporate restructuring policy to strengthen growth of its two key products — Empaveli (pegcetacoplan) and Syfovre (pegcetacoplan injection) — and curb cash burn. Shares of the company lost around 5% on Aug 29, following the announcement.
To achieve these objectives, Apellis will reduce its existing workforce by about 25% (around 225 employees), which is expected to result in up to $300 million in total cost savings through 2024.
The company will further focus on development of systematic pegcetacoplan in the phase III VALIANT study to treat primary immune-complex membranoproliferative glomerulonephritis and C3 glomerulopathy — two rare and debilitating kidney diseases. Data from the study is expected in 2024. Apart from this study, APLS has no intention to start any new clinical development of systemic pegcetacoplan.
The company plans to deprioritize development of preclinical candidates APL-1030 and APL-2006 for retinal and undisclosed indications, respectively.
Apellis is facing some safety concerns for Syfovre. It received reports of retinal vasculitis (or inflammation) following treatment with the injection in July.
The FDA approved Syfovre for the treatment of geographic atrophy secondary to age-related macular degeneration on Feb 17, 2023.
Apellis examined reported cases along with the American Society of Retina Specialists' Research and Safety in Therapeutics (ReST). These events occurred between seven and 13 days after the initial administration of the drug, with no specific lots implicated.
APLS partnered with ReST and has been closely monitoring and investigating the occurrences of retinal vasculitis. It updated the number of confirmed adverse events to eight (five occlusive and three non-occlusive). Two of these events followed injections in April, three in May and three in June. All these events occurred after the initial administration of Syfovre.
APLS is seeking to launch Syfovre in additional geographies. A regulatory application seeking approval of pegcetacoplan for the same indication as in the United States is currently under review in Europe and several countries. A decision regarding the same from the European Medicines Agency is expected in early 2024, while those from regulatory bodies in other countries are expected in the first half of 2024.
The successful approval and launch in additional geographies will add an incremental stream of revenues to APLS. Syfovre generated revenues of almost $85.7 million in the first half of 2023.
Apellis’ shares have lost 21.4% year to date compared with the industry's 11.9% decline.
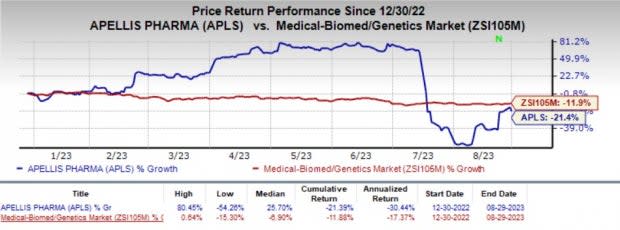
Image Source: Zacks Investment Research
On the Empaveli front, the company is taking measures to reduce associated expenses. The drug was approved for paroxysmal nocturnal hemoglobinuria by the FDA in 2021. Empaveli has shown an encouraging trend since approval, with reported revenues of almost $42.7 million in the first half of 2023.
Apellis Pharmaceuticals, Inc. Price and Consensus

Apellis Pharmaceuticals, Inc. price-consensus-chart | Apellis Pharmaceuticals, Inc. Quote
Zacks Rank & Stocks to Consider
Apellis currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same industry are ANI Pharmaceuticals ANIP, Annovis Bio ANVS and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate forANI Pharmaceuticals’ earnings has gone up from $3.31 per share to $3.73 for 2023. The bottom-line estimate has increased from $4.32 to $4.35 for 2024 during the same time frame. Shares of the company have rallied 58.0% year to date.
ANIP’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise 91.56%.
In the past 90 days, the Zacks Consensus Estimate for Annovis Bio has narrowed from a loss of $4.89 per share to a loss of $4.38 for 2023. The bottom-line estimate has narrowed from a loss of $3.18 to $2.77 for 2024 during the same time frame. Shares of the company have lost 8.6% year to date.
ANVS’ earnings beat estimates in three of the trailing four quarters and missed the mark in one, delivering an average surprise of 13.40%.
In the past 90 days, the Zacks Consensus Estimate for Corcept’s earnings has gone up from 62 cents per share to 78 cents for 2023. The bottom-line estimate has also improved from 61 cents to 83 cents for 2024 during the same time frame. Shares of the company have rallied 61.7% year to date.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
