Apellis (APLS) Down on Negative CHMP Opinion for Pegcetacoplan
Apellis Pharmaceuticals APLS announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has voted against the approval of intravitreal pegcetacoplan for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
The negative opinion of the CHMP was expected as the company previously announced a negative trend vote on the marketing authorization application (MAA) for pegcetacoplan following an oral explanation meeting in December 2023.
Per the latest press release, Apellis is currently gearing up to seek immediate re-examination of its application.
The stock fell 3.3% in the last trading session, following the disappointing regulatory update for intravitreal pegcetacoplan. Shares of the company lost 2.4% during the after-market hours. In the past year, shares of APLS have risen 21.4% against the industry’s 14.1% decline.
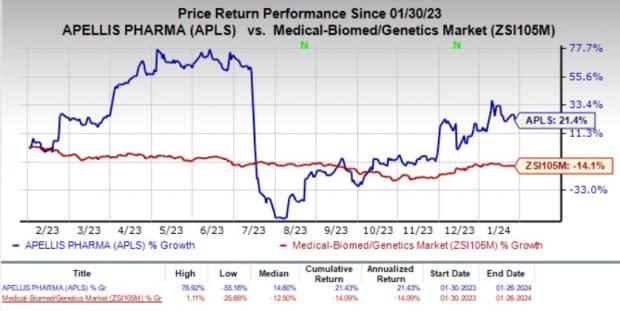
Image Source: Zacks Investment Research
The MAA submission to the EMA was based on positive 24-month results from the company’s late-stage OAKS and DERBY studies. It was observed that both every-other-month and monthly treatment with pegcetacoplan reduced GA lesion growth with increasing treatment effects over time.
The drug also demonstrated a favorable safety profile. Furthermore, treatment with pegcetacoplan preserved visual function longer in multiple post hoc phase III analyses.
GA is a progressive and irreversible form of vision loss that takes a severe toll on the patient’s quality of life. Per Apellis, more than 2.5 million people in the EU are currently living with GA.
We remind the investors that pegcetacoplan injection is currently approved in the United States under the brand name Syfovre for treating GA secondary to AMD.
Syfovre was approved by the FDA in February 2023 as the first and only treatment for GA secondary to AMD in the United States. Marketing authorization seeking applications for intravitreal pegcetacoplan for the treatment of GA is currently under review in several other countries as well. Approvals from respective regulatory bodies in different countries are expected in the first half of 2024.
Apellis also currently markets pegcetacoplan under the brand name Empaveli as a monotherapy treatment for adult patients suffering from Paroxysmal Nocturnal Hemoglobinuria (PNH).
APLS has seen a strong uptake of Empaveli in recent quarters. Empaveli is currently approved in the EU under the brand name Aspaveli (pegcetacoplan) for the treatment of adult patients with PNH who are anemic after treatment with a C5 inhibitor for at least three months.
Empaveli therapy is also being evaluated for several other rare diseases across hematology and nephrology.
Apellis recently reported strong fourth-quarter 2023 preliminary U.S. revenues, primarily driven by Syfovre’s stellar performance.
Apellis Pharmaceuticals, Inc. Price and Consensus
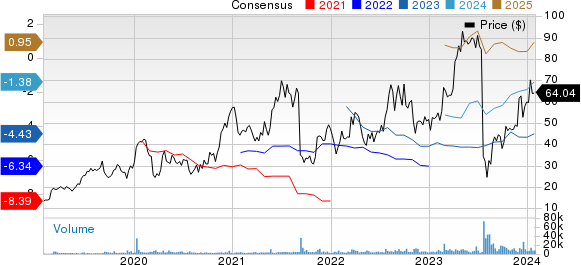
Apellis Pharmaceuticals, Inc. price-consensus-chart | Apellis Pharmaceuticals, Inc. Quote
Zacks Rank and Other Stocks to Consider
Apellis currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks from the drug/biotech industry are Puma Biotechnology, Inc. PBYI, CytomX Therapeutics CTMX and Journey Medical DERM, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has increased from 72 cents to 73 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has increased from 64 cents to 69 cents. Over the past year, shares of PBYI have risen 14.1%.
PBYI's earnings beat estimates in three of the last four quarters and missed the same in one, delivering an average surprise of 76.55%.
In the past 30 days, the Zacks Consensus Estimate for CytomX’s 2023 EPS has remained constant at 2 cents. The consensus estimate for CytomX’s 2024 loss per share is currently pegged at 6 cents. Over the past year, shares of CTMX have plunged 40.7%.
CTMX's earnings beat estimates in three of the trailing four quarters and missed the mark in one, delivering an average surprise of 45.44%.
In the past 30 days, the Zacks Consensus Estimate for Journey Medical’s 2023 loss per share has remained constant at 16 cents. During the same period, the consensus estimate for Journey Medical’s 2024 loss per share has remained constant at 69 cents. Over the past year, shares of DERM have skyrocketed 154.5%.
DERM's earnings beat estimates in one of the trailing four quarters and missed in the other three, delivering an average surprise of 118.25%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Journey Medical Corporation (DERM) : Free Stock Analysis Report
CytomX Therapeutics, Inc. (CTMX) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
