Apellis (APLS) Q2 Earnings Top, Syfovre Sales Boost Revenues
Apellis Pharmaceuticals, Inc. APLS reported a second-quarter 2023 loss of $1.02 per share, which was narrower than the Zacks Consensus Estimate of a loss of $1.35. The company reported a loss of $1.46 per share in the year-ago quarter.
Total revenues amounted to $95 million, which surpassed the Zacks Consensus Estimate of $72 million. In the year-ago quarter, the company reported revenues of $16 million. The humongous 494% jump over the year-ago quarter’s revenue figure was fueled by increasing sales of Syfovre (pegcetacoplan injection) in the reported quarter.
Despite the better-than-expected second-quarter earnings results, Apellis’ stock dropped about 20% on Monday due to safety concerns regarding its newly approved drug, Syfovre, to treat geographic atrophy (GA) secondary to age-related macular degeneration (AMD). In mid-July, Apellis received six reports of retinal vasculitis (or inflammation) following treatment with Syfovre, occurring between seven and thirteen days after the initial administration of the drug with no specific lots implicated.
The company partnered with external investigators to investigate the reported retinal vasculitis events. In a later press release, Apellis updated the number of confirmed adverse events to seven (four occlusive, three non-occlusive) and one additional reported event, which is currently being evaluated by the company. Two of these events followed injections in April, two in May and three in June.
Year to date, shares of Apellis have plunged 50.2% compared with the industry’s 11.2% fall.
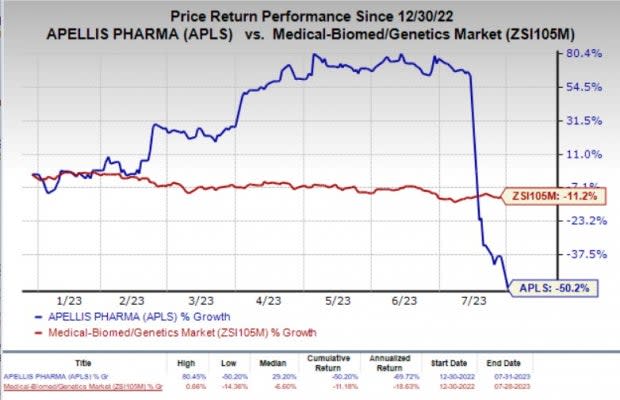
Image Source: Zacks Investment Research
Quarter in Detail
Revenues in the reported quarter included product sales of the marketed drugs — Empaveli (pegcetacoplan) and Syfovre — and licensing and other revenues, under the collaboration agreement with Sobi.
Empaveli recorded sales of $22.3 million in the reported quarter, up 42% from the year-ago quarter’s figure. Syfovre recorded its first full-quarter sales of $67.3 million. Apellis reported delivering more than 31,000 commercial vials and nearly 11,000 samples of Syfovre to doctors in the second quarter. As of Jul 29, 2023, the total number of vials of the drug delivered since the launch is reportedly more than 68,000.
In February this year, the FDA approved Syfovre for treating GA secondary to AMD. The commercial launch of the drug began in March 2023. A marketing authorization application seeking approval of pegcetacoplan for the same indication as in the United States is currently under review in Europe and several other countries. A decision regarding the same, from the European Medicines Agency, is expected in early 2024, while those from regulatory bodies in other countries are expected in the first half of 2024.
Licensing and other revenues came in at 5.3 million, up 657% from the year-ago quarter’s figure.
Research and development expenses decreased 6% to $95.7 million from the prior-year quarter’s level. This was due to a decrease in contract manufacturing expenses along with a decline in clinical and preclinical study expenses, partially offset by an increase in personnel and developmental costs.
General and administrative expenses totaled $111.4 million, up 76% from the year-ago quarter’s figure. This was driven by higher employee-related costs and an increase in professional and consulting fees.
As of Jun 30, 2023, Apellis had cash, cash equivalents and marketable securities worth $616.3 million compared with $765 million as of Mar 31, 2023. APLS expects its cash balance, combined with cash anticipated to be generated from sales of marketed products as well as Sobi reimbursements, to fund its operations into the first quarter of 2025.
Pipeline Updates
During the quarter, Apellis discontinued a phase II MERIDIAN study of systemic pegcetacoplan for amyotrophic lateral sclerosis. The decision was based on top-line results from the study which failed to meet its primary endpoint or key secondary efficacy endpoints at week 52.
The phase III VALIANT study of systemic pegcetacoplan for Immune complex membranoproliferative glomerulonephritis and C3 glomerulopathy is currently enrolling patients. Data from the study are anticipated in 2024.
Apellis’ partner, Sobi, is currently enrolling patients in its phase II study evaluating the efficacy and safety of systemic pegcetacoplan in patients with hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Data from this study are expected in 2024. Sobi is also currently enrolling patients for its phase III CASCADE study of systemic pegcetacoplan for cold agglutinin disease.
Other Updates
During the quarter, Apellis presented results from the GALE long-term extension study of Syfovre at a medical conference. Per the results, Syfovre reduced nonsubfoveal GA lesion growth by up to 45% between months 24 and 30 compared with the projected sham arm. The safety profile of the drug in the extension period was also found to be consistent with the previously reported phase III study data.
APLS has also been carrying out a comprehensive investigation into possible causes of the reported cases of retinal vasculitis. So far, the company has found no indication regarding the drug product or manufacturing issues contributed to the events. All safety events were reported to the FDA by Apellis in compliance with reporting guidelines for manufacturers.
Apellis Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
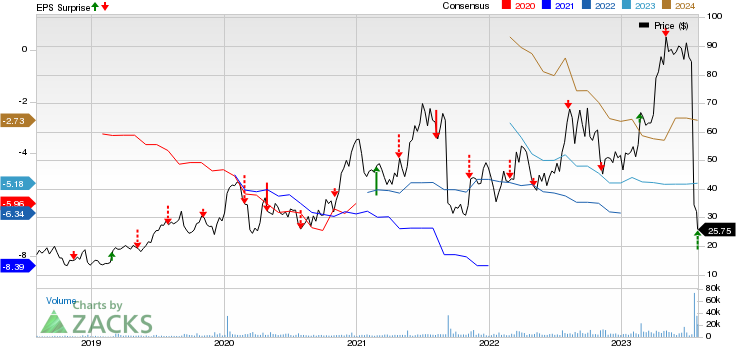
Apellis Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Apellis Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Apellis currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the overall medical sector are J&J JNJ, ADC Therapeutics ADCT and ADMA Biologics, Inc. ADMA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for J&J’s 2023 earnings per share has increased from $10.66 to $10.73. During the same period, the estimate for JNJ’s 2024 earnings per share has increased from $11.01 to $11.28. Year to date, shares of JNJ have lost 5.2%.
JNJ beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 5.58%.
In the past 90 days, the Zacks Consensus Estimate for ADC Therapeutics’ 2023 loss per share has widened from $2.60 to $2.61. During the same period, the estimate for ADC Therapeutics’ 2024 loss per share narrowed from $2.75 to $2.55. Year to date, shares of ADCT have lost 61.4%.
ADCT beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 10.70%.
In the past 90 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 14 cents to 8 cents. The consensus estimate for 2024 earnings is currently pegged at 7 cents per share. Year to date, shares of ADMA have gained 7%.
ADMA beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 19.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
ADC Therapeutics SA (ADCT) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
