Apellis (APLS) Q4 Earnings Miss, Revenues Meet Estimates
Apellis Pharmaceuticals, Inc. APLS reported fourth-quarter 2023 loss of 73 cents per share, which was wider than the Zacks Consensus Estimate of a loss of 66 cents. The company had reported a loss of $1.50 per share in the year-ago quarter.
Total revenues amounted to $146.4 million in the fourth quarter, matching the Zacks Consensus Estimate. In the year-ago quarter, the company had reported revenues of $22.6 million.
The top line jumped almost 545% year over year owing to higher sales of Syfovre (pegcetacoplan injection) in the reported quarter.
Syfovre was approved for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration by the FDA in February 2023.
In January 2024, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) voted against the approval of Syfovre for the treatment of GA secondary to age-related macular degeneration.
The negative opinion of the CHMP was expected as the company previously announced a negative trend vote on the marketing authorization application (MAA) for Syfovre following an oral explanation meeting in December 2023.
Apellis’ shares have rallied 8.4% in the past year against the industry’s decline of 7.2%.
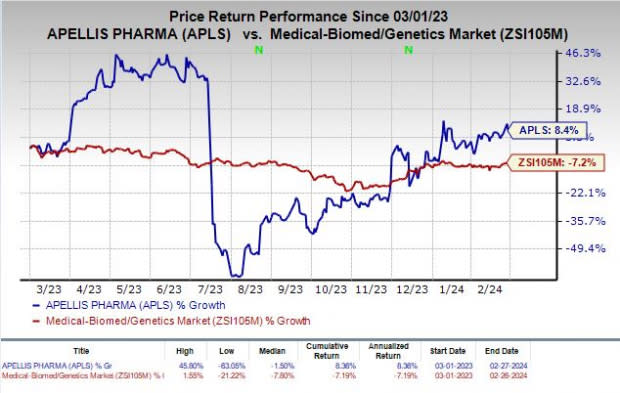
Image Source: Zacks Investment Research
Quarter in Detail
Revenues in the reported quarter included product sales of the marketed drugs — Empaveli (pegcetacoplan) and Syfovre — and licensing and other revenues, under the collaboration agreement with Sobi.
Syfovre recorded sales of $114.3 million in the fourth quarter, increasing around 51.4% sequentially owing to continued strong demand.
Syfovre sales marginally beat our model estimate of $114 million.
Apellis delivered more than 55,000 commercial vials and nearly 6,400 samples of Syfovre to doctors in the fourth quarter. As of Dec 31, 2023, the total number of doses of the drug delivered since launch was reportedly more than 160,000.
The potential approval and successful launch of Syfovre in additional geographies will add an incremental stream of revenues to APLS.
In October 2023, APLS received the permanent J-code for Syfovre, which is likely to help the company streamline billing and reimbursement of the medicine.
Empaveli recorded sales of $24.4 million in the reported quarter, up 23.8% from the year-ago quarter’s figure, owing to the increasing number of patient switches from AstraZeneca’s Ultomiris (eculizumab).
Empaveli sales, too, were almost in line with our model estimate of $24.3 million.
Empaveli is approved in the United States for the treatment of paroxysmal nocturnal hemoglobinuria. The drug is also approved in Europe under the brand name Aspaveli for the same indication.
Licensing and other revenues came in at $7.7 million, up 156.7% from the year-ago quarter’s figure.
Research and development expenses decreased 30.3% from the prior-year quarter’s level to $69.3 million. This was due to a decrease in program-specific external costs and other external costs.
General and administrative expenses totaled $141.7 million, up 67.8% from the year-ago quarter’s figure. This was driven by higher employee-related costs and higher office costs.
As of Dec 31, 2023, Apellis had cash, cash equivalents and marketable securities worth $351.2 million compared with $452.4 million as of Sep 30, 2023. APLS expects its cash balance, combined with cash anticipated from sales of marketed products, to be enough to fund its operations in the foreseeable future.
Full-Year Results
In 2023, Apellis generated revenues of $396.6 million, compared with $75.4 million reported in 2022.
In the same period, the company recorded a loss of $4.45 per share compared with a loss of $6.15 in 2022.
Pipeline & Other Updates
Following the CHMP’s negative opinion for Syfovre in Europe, Apellis is initiating a re-examination of the MAA for Syfovre with the EMA. A final opinion from the CHMP is expected in the second quarter of 2024. If positive, a decision from the European Commission is anticipated in the third quarter.
The FDA approved Empaveli Injector as a single-use, on-body device to enhance the self-administration of Empaveli in October 2023. The injector aims to provide patients with greater mobility and convenience in managing PNH.
The Empaveli Injector is the first of its kind, offering a high-volume (20ml), subcutaneous on-body drug delivery system that can simplify the process of self-administration. APLS expects the injector to drive sales of the product.
Apellis is currently enrolling patients in the phase III VALIANT study evaluating systemic pegcetacoplan for treating immune complex membranoproliferative glomerulonephritis (IC-MPGN) and C3 glomerulopathy. Data from the study are anticipated in mid-2024.
Apellis’ partner, Sobi, is currently enrolling patients in its phase II study evaluating the efficacy and safety of systemic pegcetacoplan in patients with hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Data from this study are expected later in 2024. Sobi is also currently enrolling patients for its phase III CASCADE study of systemic pegcetacoplan for cold agglutinin disease.
Apellis Pharmaceuticals, Inc. Price, Consensus and EPS Surprise

Apellis Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Apellis Pharmaceuticals, Inc. Quote
Zacks Rank & Stocks to Consider
Apellis currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Indivior PLC INDV Vanda Pharmaceuticals Inc. VNDA and Puma Biotechnology, Inc. PBYI, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Indivior’s 2024 earnings per share have improved from $1.83 to $1.95. In the past year, shares of INDV have risen 14.3%.
Indivior’s earnings beat estimates in each of the trailing three quarters. INDV delivered an average earnings surprise of 48.06%.
In the past 60 days, the Zacks Consensus Estimate for Vanda Pharmaceuticals’ 2024 bottom line has improved from a loss of 46 cents per share to earnings of 1 cent. In the past year, shares of VNDA have plunged 28.7%.
Vanda Pharmaceuticals’ earnings beat estimates in each of the trailing three quarters. VNDA delivered an average earnings surprise of 92.88%.
In the past 60 days, estimates for Puma Biotechnology’s 2024 earnings per share have improved from 69 cents to 71 cents. In the past year, shares of PBYI have risen 67.4%.
Earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Vanda Pharmaceuticals Inc. (VNDA) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
Indivior PLC (INDV) : Free Stock Analysis Report
