Applied Therapeutics Inc (APLT) Announces Year-End 2023 Financial Results and Clinical Progress
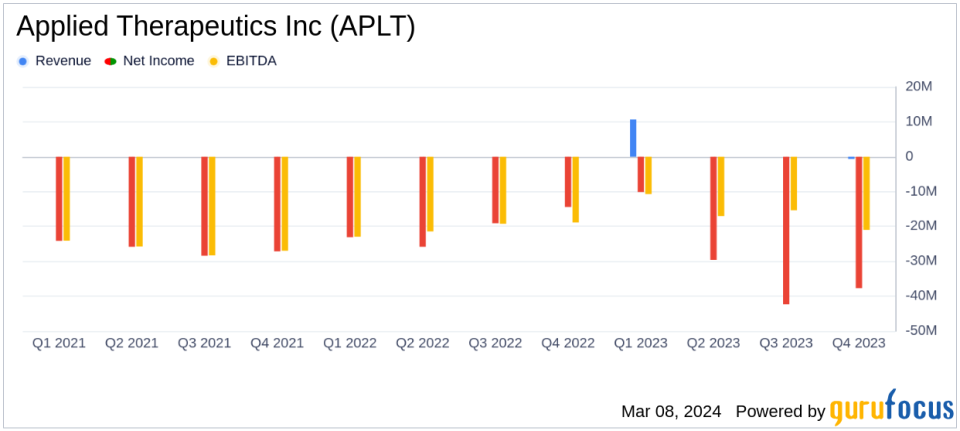
Revenue: Reported total revenue of $9.99 million for the year ended December 31, 2023.
Net Loss: Incurred a net loss of $119.76 million, or $1.42 per share, for the year ended December 31, 2023.
Research and Development Expenses: R&D expenses totaled $53.91 million for the year ended December 31, 2023.
General and Administrative Expenses: G&A expenses were $20.62 million for the year ended December 31, 2023.
Cash Position: Cash and cash equivalents stood at $49.90 million as of December 31, 2023.
Clinical Milestones: NDA for govorestat accepted and granted Priority Review by FDA, with a PDUFA target action date of August 28, 2024.
Financing: Completed a $100 million Private Placement, extending cash runway into 2026.
On March 6, 2024, Applied Therapeutics Inc (NASDAQ:APLT) released its 8-K filing, detailing the financial results for the fourth quarter and full year ended December 31, 2023. The clinical-stage biopharmaceutical company, known for developing novel drug candidates against validated molecular targets in indications of high unmet medical need, has reported significant clinical and regulatory progress over the past year.
Financial Performance and Clinical Developments
Applied Therapeutics' financial results reflect a year of strategic execution and clinical advancements. The company reported a total revenue of $9.99 million for the year, primarily from license revenue. Despite these revenues, the company incurred a net loss of $119.76 million, or $1.42 per share, which is attributed to ongoing investments in research and development (R&D) and general and administrative (G&A) expenses. R&D expenses amounted to $53.91 million, a slight decrease from the previous year, while G&A expenses were reported at $20.62 million.
The company's balance sheet was strengthened by a $100 million Private Placement, which is expected to extend its cash runway into 2026. As of December 31, 2023, Applied Therapeutics had cash and cash equivalents of $49.90 million, compared to $16.66 million at the end of 2022.
Strategic Highlights and Future Outlook
Dr. Shoshana Shendelman, Founder, CEO, and Chair of the Board, highlighted the acceptance and Priority Review designation of the New Drug Application (NDA) for govorestat for the treatment of Classic Galactosemia by the FDA. The Prescription Drug User Fee Act (PDUFA) target action date is set for August 28, 2024. Additionally, the Marketing Authorization Application (MAA) is under review by the European Medicines Agency (EMA), with a decision expected in the fourth quarter of 2024.
"Weve made significant clinical and regulatory progress, particularly with the NDA acceptance and MAA validation for govorestat for the treatment of Galactosemia, achieving key milestones for our rare disease pipeline. Additionally, we believe that the recent positive data from the interim analysis of the INSPIRE study in SORD Deficiency confirms the role of sorbitol as a key driver of disease progression, and we plan to request a pre-NDA meeting with the FDA," said Shoshana Shendelman, PhD, Founder, Chief Executive Officer, and Chair of the Board. "As Applied enters into this next stage of growth, we are poised for continued value generation across our rare disease pipeline, supported by our recent financing and bolstered cash position."
The company's lead drug candidate, govorestat, is a central nervous system penetrant Aldose Reductase Inhibitor (ARI) for the treatment of rare metabolic diseases. Applied Therapeutics is also advancing AT-001 for Diabetic Cardiomyopathy and AT-003 for Diabetic Retinopathy, further diversifying its pipeline.
Applied Therapeutics' commitment to addressing unmet medical needs in rare diseases, supported by a solid financial foundation and promising clinical data, positions the company for potential success in the coming years. Investors and stakeholders will be closely monitoring the FDA's decision on govorestat and the company's continued progress in its pipeline development.
For more detailed information on Applied Therapeutics Inc's financial results and strategic initiatives, please refer to the full 8-K filing.
Investors interested in the latest updates and analyses on Applied Therapeutics Inc (NASDAQ:APLT) and other value investment opportunities are encouraged to visit GuruFocus.com for comprehensive financial news and insights.
Explore the complete 8-K earnings release (here) from Applied Therapeutics Inc for further details.
This article first appeared on GuruFocus.
