Aquestive (AQST) Up as Anaphylm Pivotal Study Meets Goals
Aquestive Therapeutics, Inc. AQST shares jumped 16% on Mar 15 after the company achieved the primary and secondary endpoints in the two-part phase III pivotal pharmacokinetic clinical study of Anaphylm (epinephrine) Sublingual Film to treat severe allergic reactions. The stock price surge was also likely boosted by findings from the FDA Type C meeting.
AQST’s Anaphylm is a non-invasive oral epinephrine product candidate. It has the potential to offer greater convenience of use compared with autoinjectors, such as EpiPen and Auvi-Q, for the emergency treatment of severe allergic reactions, including anaphylaxis.
Per data from the pivotal phase III study, Anaphylm was non-inferior to the leading autoinjectors immediately following administration. Anaphylm was also observed to be consistently faster than the leading autoinjectors on the grounds of time to maximum concentration (Tmax).
Tmax is an important metric used by clinicians to gauge the rate of absorption of epinephrine at the first sign of symptoms, which is considered critical while treating severe allergic reactions, including anaphylaxis.
Per Aquestive, Anaphylm’s Tmax has not been witnessed among the alternate delivery options under development.
Furthermore, it was also observed that in the pivotal study, Anaphylm exposure levels, as measured by area under the curve (AUC), were comparable to autoinjectors for 30 minutes after dosing. AUC is a metric used to measure the overall exposure to a drug. Per observations, Anaphylm maintained a similar level of epinephrine exposure compared with intra-muscularly injected epinephrine during those 30 minutes.
The candidate was overall well-tolerated and no treatment-related adverse events were observed.
In the past year, shares of AQST have skyrocketed 668.9% against the industry's 4.6% decline.
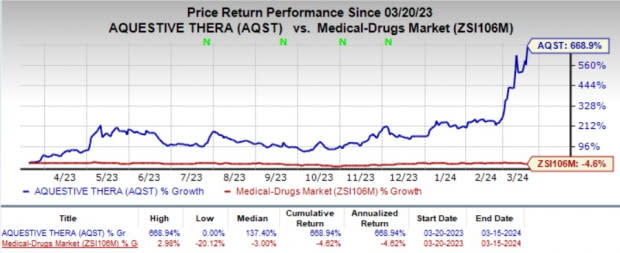
Image Source: Zacks Investment Research
In the same press release, Aquestive reported that it has received positive feedback from the FDA at a Type C meeting, which provided the company with a clear idea of the remaining clinical development steps necessary for a pre-new drug application (NDA) meeting with the FDA in the second half of the year. The company plans to file an NDA for Anaphylm to treat severe allergies by the end of 2024, subject to a positive pre-NDA meeting.
The FDA has also recommended that Aquestive should begin its pediatric study evaluating Anaphylm for the same indication after the completion of the remaining adult studies.
Aquestive is aligned with the recommendations made by the regulatory body.
Please note that the brand name Anaphylm is approved by the FDA on a conditional basis, the final approval of which is subject to the regulatory body’s approval of the product candidate.
Aquestive Therapeutics, Inc. Price and Consensus
Aquestive Therapeutics, Inc. price-consensus-chart | Aquestive Therapeutics, Inc. Quote
Zacks Rank and Stocks to Consider
Aquestive currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are ADMA Biologics ADMA, FibroGen FGEN and Adicet Bio, Inc. ACET. While ADMA sports a Zacks Rank #1 (Strong Buy), FGEN and ACET carry a Zacks Rank #2 (Buy) each at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2024 earnings per share (EPS) has increased from 22 cents to 30 cents. During the same period, the estimate for ADMA’s 2025 EPS has increased from 32 cents to 50 cents. In the past year, shares of ADMA have surged 92.3%.
ADMA beat estimates in three of the trailing four quarters and matched in one, delivering an average earnings surprise of 85%.
In the past 30 days, the Zacks Consensus Estimate for FibroGen’s 2024 loss per share has narrowed from $1.14 to $1.09. During the same period, the estimate for FibroGen’s 2025 loss per share is pegged at 6 cents. In the past year, shares of FGEN have plunged 89.7%.
FGEN beat estimates in two of the trailing four quarters, missing the mark on the other two occasions, delivering an average negative surprise of 2.26%.
In the past 30 days, the Zacks Consensus Estimate for Adicet Bio’s 2023 loss per share has remained constant at $3.39. During the same period, the consensus estimate for Adicet’s 2024 loss per share has remained constant at $1.81. In the past year, shares of ACET have plunged 73.4%.
ACET beat estimates in two of the trailing four quarters, missing the mark on the other two occasions, delivering an average negative surprise of 8.36%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Adicet Bio, Inc. (ACET) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
FibroGen, Inc (FGEN) : Free Stock Analysis Report
Aquestive Therapeutics, Inc. (AQST) : Free Stock Analysis Report
