Arcturus Therapeutics Reports Fiscal Year 2023 Financial Results and Pipeline Developments
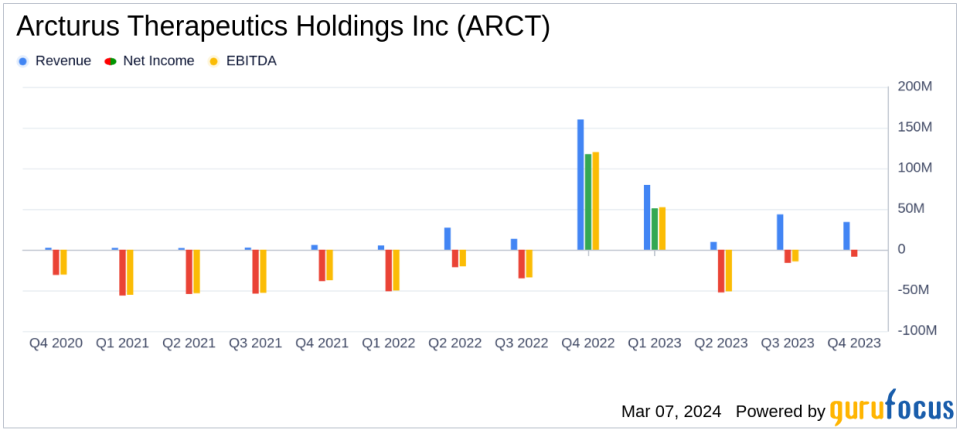
Revenue: Year-end revenue decreased to $169.9 million in 2023 from $206.0 million in 2022.
Operating Expenses: Increased to $245.0 million in 2023 from $193.8 million in 2022.
Net Loss: Reported a net loss of $26.6 million in 2023, compared to a net income of $9.3 million in 2022.
Cash Position: Cash, cash equivalents, and restricted cash totaled $348.9 million at the end of 2023.
Pipeline Progress: Advancements in ARCT-032 and ARCT-810, and initiation of new vaccine programs for Lyme Disease and Gonorrhea.
Regulatory Milestones: Orphan Drug Designation granted for ARCT-032 by the U.S. FDA and the European Commission.
Commercialization Efforts: Kostaive set to launch in Japan, marking the world's first self-amplifying mRNA product approval.
On March 7, 2024, Arcturus Therapeutics Holdings Inc (NASDAQ:ARCT) released its 8-K filing, detailing the financial results for the fourth quarter and fiscal year ended December 31, 2023, and providing updates on its pipeline progress. Arcturus, a prominent RNA medicines company, is focused on developing vaccines and therapeutics for infectious diseases, liver, and respiratory rare diseases. The company's pipeline includes notable candidates such as LUNAR-OTC, LUNAR-CF, and LUNAR-COV19.
Financial Performance and Challenges
Arcturus reported a decrease in year-end revenue to $169.9 million in 2023, down from $206.0 million in the previous year. This decline was primarily due to the discontinuation of collaboration agreements with Vinbiocare and Janssen. Despite this, revenue from CSL slightly increased by $3.0 million compared to 2022, and progress with the BARDA grant agreement led to an $8.8 million increase in revenue. However, the company faced a significant net loss of $26.6 million, or ($1.00) per diluted share, a stark contrast to the net income of $9.3 million, or $0.35 per diluted share, reported in the previous year.
The increase in operating expenses to $245.0 million in 2023 from $193.8 million in 2022 reflects the company's investment in research and development for its CSL and BARDA programs, as well as internal programs targeting OTC and Cystic Fibrosis. These investments are crucial for Arcturus as it aims to bring innovative RNA-based therapies to market, which is particularly important in the biotechnology industry where the development cycle is long and capital-intensive.
Financial Achievements and Importance
Despite the challenges, Arcturus has made significant strides in extending its cash runway to the first quarter of 2027, thanks to disciplined cost management and the progression of the CSL collaboration. This financial stability is vital for the company to continue its research and development efforts without the immediate need for additional funding. Furthermore, the company's collaboration with CSL Seqirus and the anticipated launch of Kostaive in Japan represent important milestones in commercializing the world's first self-amplifying mRNA product.
Key Financial Metrics and Commentary
Arcturus' financial position is highlighted by its cash, cash equivalents, and restricted cash totaling $348.9 million at the end of 2023. The company's President & CEO, Joseph Payne, expressed excitement about the pipeline progress and commercialization efforts, particularly the approval of Kostaive. Payne noted the stronger, broader, and more durable immune response induced by Kostaive compared to an approved conventional mRNA vaccine.
"We are especially pleased to announce the U.S. FDA and the European Commission recently granted Orphan Drug Designation for ARCT-032, an inhaled mRNA therapeutic candidate for individuals with cystic fibrosis," said Joseph Payne.
Chief Financial Officer Andy Sassine added:
"I am pleased to announce the cash runway was extended to the first quarter 2027 due to disciplined cost management and progression of the CSL collaboration."
These commentaries emphasize the company's strategic focus on advancing its pipeline and securing a strong financial foundation for future growth.
Analysis of Company Performance
Arcturus' performance in 2023 reflects a company in a transition phase, balancing the costs of advancing its pipeline with the need to manage its financial resources effectively. The extended cash runway and regulatory milestones achieved for ARCT-032 and ARCT-810 are positive indicators of the company's potential to deliver on its pipeline promises. However, the net loss underscores the inherent risks and costs associated with drug development in the biotechnology sector. The company's ability to navigate these challenges while progressing its pipeline will be critical to its long-term success.
For more detailed information on Arcturus Therapeutics Holdings Inc (NASDAQ:ARCT)'s financial results and pipeline updates, investors are encouraged to review the full 8-K filing.
Explore the complete 8-K earnings release (here) from Arcturus Therapeutics Holdings Inc for further details.
This article first appeared on GuruFocus.
