Arcutis (ARQT) Up on FDA Nod for Zoryve in Seborrheic Dermatitis
Arcutis Biotherapeutics, Inc. ARQT announced that the FDA has approved the new drug application (NDA) for Zoryve (roflumilast) topical foam 0.3% for the treatment of seborrheic dermatitis in patients aged nine years and above. Shares of the company were up 4.1% on Dec 18, following the announcement of the news on Dec 15.
Seborrheic dermatitis is a skin condition that usually affects the scalp. Zoryve once-daily steroid-free foam is effective, safe, well-tolerated for use on all affected areas of the body, including hair-bearing areas.
Following the latest nod, Zoryve foam is now the first drug to be approved by the FDA to treat seborrheic dermatitis with a new mechanism of action in more than two decades. Arcutis plans to launch Zoryve foam in the United States by the end of January 2024.
The above approval was based on positive data from two studies – the STRATUM and the phase II (Trial 203) study.
Data from the STRATUM study showed that around 80% of individuals treated with Zoryve foam 0.3% achieved Investigator Global Assessment (IGA) success rate at week 8 compared to 58% in the vehicle arm, thereby meeting the primary endpoint. Treatment with Zoryve foam also led to statistically significant improvement over vehicle arm on all secondary endpoints, including itch, scaling and erythema (redness) in the STRATUM study.
Meanwhile, in the phase II (Trial 203) study, 73% of individuals treated with Zoryve foam achieved IGA success compared with 40.8% in the vehicle arm.
Following the latest FDA nod for Zoryve foam in seborrheic dermatitis, the company is planning to submit a supplemental new drug application (sNDA) seeking approval for Zoryve foam to treat scalp and body psoriasis.
Shares of Arcutis have plunged 82.1% in the past year compared with the industry’s decline of 18.3%.
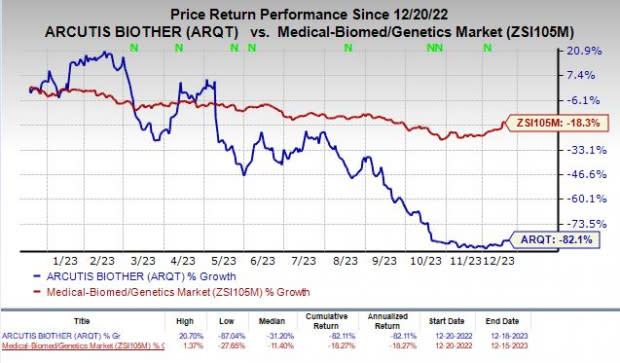
Image Source: Zacks Investment Research
Zoryve cream formulation is approved in the United States for the treatment of plaque psoriasis in patients aged 12 years and above. The FDA approved the sNDA for Zoryve cream 0.3% to treat patients in the age group of 6-11 years suffering from plaque psoriasis in October 2023.
In the first nine months of 2023, Arcutis earned $15.6 million from the sale of the drug.
Another sNDA seeking label expansion of roflumilast cream 0.15% to treat patients aged six years and older with atopic dermatitis is currently under review in the United States. A decision from the FDA is expected on Jul 07, 2024.
Zacks Rank & Other Stocks to Consider
Arcutis currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the healthcare sector are Journey Medical Corporation DERM, Entrada Therapeutics, Inc. TRDA and Puma Biotechnology, Inc. PBYI, each sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Journey Medical’s 2023 loss per share have narrowed from $1.28 to 16 cents. Meanwhile, loss per share estimates for 2024 have narrowed from 41 cents to 35 cents. In the past year, shares of DERM have surged 533.6%.
Earnings of Journey Medical beat estimates in one of the last four quarters while missing the same on the remaining three occasions. DERM delivered a four-quarter earnings surprise of 118.25%, on average.
In the past 60 days, estimates for Entrada Therapeutics’ 2023 loss per share have narrowed from $2.07 to 9 cents. Meanwhile, loss per share estimates for 2024 have narrowed from $2.35 to $2.04. In the past year, shares of TRDA have decreased 8.9%.
Earnings of Entrada Therapeutics beat estimates in three of the last four quarters while missing the same on the remaining occasion. TRDA delivered a four-quarter average earnings surprise of 70.68%.
In the past 60 days, estimates for Puma Biotechnology’s 2023 earnings per share have improved from 67 cents to 72 cents. During the same period, earnings per share estimates for 2024 have moved up from 55 cents to 64 cents. In the past year, shares of PBYI have lost 22.2%.
Earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Journey Medical Corporation (DERM) : Free Stock Analysis Report
Arcutis Biotherapeutics, Inc. (ARQT) : Free Stock Analysis Report
Entrada Therapeutics, Inc. (TRDA) : Free Stock Analysis Report
