ASLAN (ASLN) Up 161% on Upbeat Initial Data From Eczema Study
ASLAN Pharmaceuticals Ltd. ASLN skyrocketed 160.8% on Mar 11 due to encouraging preliminary data reaffirming the potential of its investigational candidate, eblasakimab, to effectively treat atopic dermatitis (AD, also called eczema) patients. The intended patient population includes those previously treated with Sanofi SNY and Regeneron’s REGN blockbuster drug Dupixent (dupilumab).
Eblasakimab, a novel monoclonal antibody, is an inhibitor of the IL-13 receptor sub-unit of the Type 2 receptor, which is responsible for several allergic and inflammatory diseases.
The company is currently evaluating the safety and efficacy of eblasakimab in a phase II TREK-DX study in the AD patient population. The TREK study is expected to enroll approximately 75 patients who have discontinued treatment with Sanofi/Regeneron’s Dupixent for any reason, including inadequate control of AD, loss of access or an adverse event.
The preliminary data readout is from 22 enrolled patients who have a baseline Eczema Area and Severity Index (EASI) score of 16. Out of the 22 eczema patients, 17 completed the 16-week treatment period and five discontinued before completion. Among these 17 patients, 10 (45%) achieved at least a 90% reduction in EASI score and 11 (50%) achieved clear or almost clear skin after 16 weeks.
Furthermore, out of the nine patients who previously had an inadequate response to Sanofi/Regeneron’s Dupixent, five achieved at least a 90% reduction in EASI score and five patients attained clear or almost clear skin.
Such preliminary data from the TREK-DX study suggest that eblasakimab, with its unique mechanism of action, could have the potential to be effective in AD patients who do not achieve an adequate response to the highly successful medicine, Dupixent.
In the past year, shares of ASLAN have plunged 36.3% compared with the industry’s 4.8% decline.
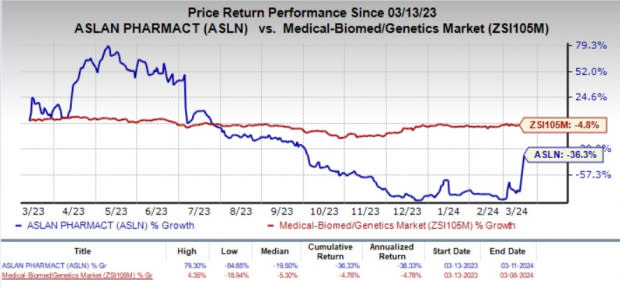
Image Source: Zacks Investment Research
The company also reported that eblasakimab has been well tolerated to date, with no new safety signals. Additionally, there were no reports of conjunctivitis or injection site reactions. Top-line data from the full dataset of the TREK-DX study is anticipated by 2024-end.
In the same press release, ASLAN announced a protocol update in the ongoing TREK-DX study, based on the findings from its TREK-AD study, which evaluated the efficacy and safety of eblasakimab as monotherapy in biologic-naïve adult patients with moderate-to-severe AD who are candidates for systemic therapy.
Per the company, findings of the TREK-AD study highlighted the changing AD patient population in the United States, which called for tightening of the inclusion criteria to enroll patients with a baseline EASI score of at least 18, instead of 16.
We note that the preliminary review of data was from the 22 patients in the TREK-DX study who were enrolled under the original inclusion criteria of EASI-16.
The updated protocol also requires the confirmation of baseline EASI scores from an independent reviewer.
ASLAN has reported that the TREK-DX study sites in the United States have now begun enrolling AD patients in compliance with the updated criteria and additional sites in the EU are on track to open in the first half of 2024.
ASLAN Pharmaceuticals Ltd. Price and Consensus
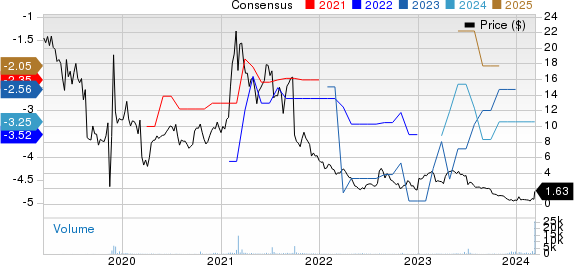
ASLAN Pharmaceuticals Ltd. price-consensus-chart | ASLAN Pharmaceuticals Ltd. Quote
Sanofi and Regeneron’s Dupixent is presently approved in several countries, including the United States and EU, for five type II inflammatory diseases, namely severe chronic rhinosinusitis with nasal polyposis, severe asthma, moderate-to-severe atopic dermatitis, eosinophilic esophagitis and prurigo nodularis.
Dupixent is one of the key top-line drivers of Sanofi, currently annualizing at close to €11.0 billion in sales after around seven years on the market. Sanofi expects Dupixent to achieve more than €13 billion in sales in 2024
SNY markets Dupixent in partnership with Regeneron. While sales are recorded by Sanofi, REGN records its share of profits/losses in connection with global sales of the drug.
Zacks Rank and Stock to Consider
ASLAN currently carries a Zacks Rank #3 (Hold).
A better-ranked stock from the drug/biotech industry is ADMA Biologics ADMA, sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2024 earnings per share (EPS) has increased from 22 cents to 30 cents. During the same period, the estimate for ADMA’s 2025 EPS has increased from 32 cents to 50 cents. Over the past year, shares of ADMA have surged 94.3%.
ADMA beat estimates in three of the trailing four quarters and matched in one, delivering an average earnings surprise of 85%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
ASLAN Pharmaceuticals Ltd. (ASLN) : Free Stock Analysis Report
