AstraZeneca (AZN) Gets EU Nod to Expanded Use of 2 Cancer Drugs
AstraZeneca PLC AZN, along with partner Merck MRK, announced that the European Commission (EC) has approved their blockbuster PARP inhibitor Lynparza (olaparib) for the first-line treatment of metastatic castration-resistant prostate cancer (mCRPC).
In Europe, Lynparza is now approved in combination with J&J’s JNJ prostate cancer drug Zytiga (abiraterone) and corticosteroid prednisone or prednisolone for mCRPC in adult men for whom chemotherapy is not clinically indicated.
Following the EC’s nod, Lynparza, in combination with JNJ’s Zytiga, becomes the first PARP inhibitor to be approved in combination with a new hormonal agent for patients in the mCRPC setting in Europe.
The nod from EC was based on data from the phase III study, PROpel. In the study, Lynparza plus J&J’s Zytiga and prednisone or prednisolone reduced the risk of disease progression or death by 34% versus J&J’s Zytiga alone. Median radiographic progression-free survival was 24.8 months in the Lynparza plus Zytiga arm versus 16.6 months for Zytiga alone.
The EU approval was expected as in November, the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) rendered a positive opinion recommending approval of Lynparza for the above indication.
However, in the United States, earlier this month, the FDA extended the review period for AZN and MRK’s supplemental new drug application (“sNDA”) seeking approval for Lynparza in a similar mCRPC indication by three months. Earlier, the FDA decision on this sNDA was expected in the fourth quarter.
Lynparza is approved for four cancer types, namely ovarian, breast, prostate and pancreatic. It is also being evaluated in an earlier-line setting for the approved cancer indications as well as some other cancer types. AstraZeneca markets Lynparza in partnership with Merck.
Shares of AstraZeneca have rallied 16.2% so far this year compared with the industry’s rise of 10.7%.
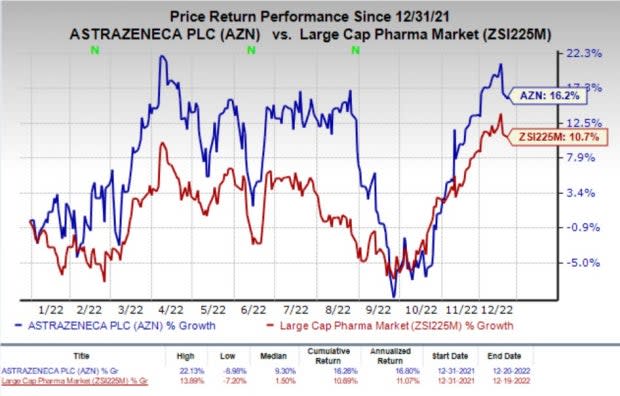
Image Source: Zacks Investment Research
In a separate press release, AZN announced that the EC has approved its PD-L1 inhibitor Imfinzi (durvalumab) in combination with chemotherapy (gemcitabine plus cisplatin) for the first-line treatment of unresectable or metastatic biliary tract cancer (BTC). BTC is a group of rare and aggressive cancers that occur in the bile ducts and gallbladder.
In November, the CHMP rendered a positive opinion recommending approval of the Imfinzi combo for the given indication.
Data from an interim analysis of the phase III TOPAZ-1 study showed that Imfinzi plus chemotherapy led to a 20% reduction in the risk of death versus chemotherapy alone. Updated data from the same showed that Imfinzi plus chemotherapy led to a 24% reduction in the risk of death versus chemotherapy alone.
In the United States, Imfinzi plus chemotherapy was approved for treating locally advanced or metastatic BTC in September.
Zacks Rank & Stock to Consider
AstraZeneca currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the large-cap pharma sector is Sanofi SNY, carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Earnings estimates for Sanofi have been revised upward 6.2% for 2022 and 2.1% for 2023 in the past 60 days.
Earnings of Sanofi have surpassed estimates in all the trailing four quarters. SNY witnessed an earnings surprise of 9.50% on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
