AstraZeneca (AZN) Gets FDA CRL for Ultomiris in New Indication
AstraZeneca AZN announced that the FDA has issued a complete response letter to its regulatory application seeking approval of Ultomiris for a new rare and debilitating autoimmune disease.
The supplemental biologics license application (sBLA) sought approval for long-acting C5 complement inhibitor Ultomiris for the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-aquaporin-4 antibody positive.
The sBLA was based on data from the CHAMPION-NMOSD phase III study. However, the FDA has neither requested any additional analysis of data from the CHAMPION-NMOSD phase III study nor raised any concerns about the efficacy and safety data from the study. It rather requests modifications in the Ultomiris Risk Evaluation and Mitigation Strategy safety program.
Year to date (YTD), the stock has declined 0.3% against the industry’s 7.2% rise.
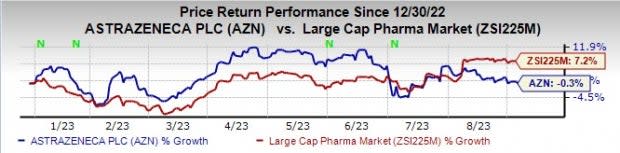
Image Source: Zacks Investment Research
Ultomiris was approved for the same rare disease indication in the EU in May this year and is also approved in Japan for NMOSD.
The drug is presently approved for treating three indications — generalized Myasthenia Gravis (gMG), paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome in the United States.
Ultomiris was added to AstraZeneca’s portfolio with the July 2021 acquisition of Alexion.
The drug recorded sales of $1.36 billion in the first half of 2023, up 64% at the constant exchange rate. Ultomiris sales are benefitting from label expansion for the gMG indication, expansion into new markets and continued conversion from Alexion’s older drug, Soliris
Zacks Rank & Stocks to Consider
Currently, AstraZeneca has a Zacks Rank #3 (Hold).
AstraZeneca PLC Price and Consensus
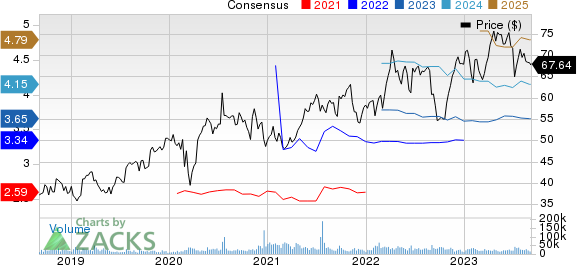
AstraZeneca PLC price-consensus-chart | AstraZeneca PLC Quote
Some better-ranked biotech companies are Exelixis EXEL, Dynavax Technologies Corporation DVAX and Corcept Therapeutics CORT, each with a Zacks Rank of 2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for Exelixis’ 2023 earnings per share have risen from 89 cents to 98 cents per share. During the same period, earnings per share estimates for 2024 have risen from $1.31 to $1.36. Year to date, shares of Exelixis have risen 38.8%.
Earnings of Exelixis beat estimates in three of the last four quarters, delivering an earnings surprise of 18.62%, on average.
In the past 60 days, estimates for Dynavax Technologies’ 2023 loss per share have narrowed from 56 cents to 24 cents, while those for 2024 have improved from a loss of 24 cents to earnings of 2 cents. Shares of Dynavax Technologies have risen 33.7% YTD.
Earnings of Dynavax Technologies beat estimates in two of the last four quarters and missed the mark on two occasions. On average, the company witnessed an earnings surprise of 25.78% over the trailing four quarters.
In the past 60 days, the Zacks Consensus Estimate for Corcept’s earnings has gone up from 62 cents per share to 78 cents for 2023. The bottom-line estimate has also improved from 61 cents to 83 cents for 2024 during the same time frame. Shares of the company have rallied 55.8% year to date.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
