AstraZeneca (AZN) & Quell to Co-Develop Autoimmune Cell Therapies
AstraZeneca AZN announced entering into a collaboration, exclusive option and license agreement with Quell Therapeutics to co-develop several engineered T-regulator (Treg) cell therapies in major autoimmune indications. This collaboration agreement gives AstraZeneca access to Quell’s proprietary toolbox of Treg cell engineering modules, including its innovative Foxp3 Phenotype Lock, for the development of Treg cell therapy candidates, as potential cures in type 1 diabetes (T1D) and inflammatory bowel disease (IBD) indications.
Per the terms of the agreement, Quell is entitled to an upfront payment of $85 million, comprising a significant cash payment and an equity investment, from AstraZeneca. If successful, Quell is also eligible to receive more than $2 billion upon achieving further development and commercialization milestones along with tiered royalty payments.
Additionally, Quell retains an option, which can be exercised either after approval of an investigational new drug application or at the end of the phase I/II study, to co-develop Treg cell therapies from the T1D program with AstraZeneca in the United States. Exercising the option will make Quell eligible for additional milestone payments and increased royalties on U.S. net sales.
So far this year, shares of AstraZeneca have gained 9.2% compared with the industry’s brisk 0.8% rise.
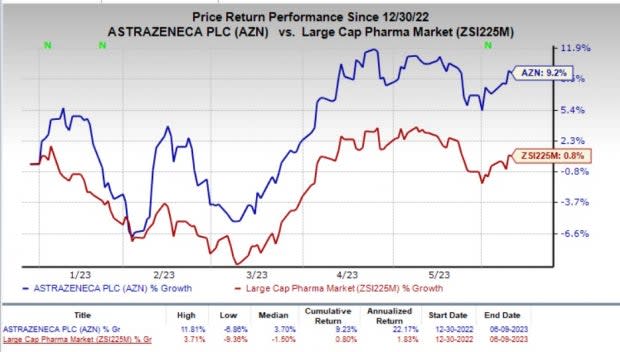
Image Source: Zacks Investment Research
Per the agreement, AstraZeneca will have the option to further development and commercialization of successful clinical candidates in T1D and IBD.
In a separate press release, AstraZeneca announced positive results from its pivotal phase III ALPHA study, evaluating danicopan, an investigational and first-in-class oral Factor D inhibitor, as an add-on to standard of care (SOC) C5 inhibitor therapy Ultomiris (ravulizumab) or Soliris (eculizumab).
The phase III ALPHA study met its primary efficacy endpoint of a statistically significant and clinically meaningful increase in haemoglobin levels from baseline to week 12 and maintained disease control in patients with paroxysmal nocturnal haemoglobinuria (PNH) who experience clinically significant extravascular haemolysis (EVH) compared with placebo plus SOC C5 inhibitor therapy. Results showed that 59.5% of significantly more patients treated with danicopan (59.5%) versus placebo (0%) experienced an improvement in haemoglobin of ≥2 g/dL at week 12 in the absence of transfusion.
The danicopan plus Ultomiris or Soliris arm of the ALPHA study also met all of its corresponding secondary endpoints with statistical significance at week 12. Danicopan reduced fatigue and anaemia as well as the need for transfusions in PNH patients with clinically significant EVH compared with placebo plus C5 inhibition.
Additionally, danicopan was generally well tolerated during the phase III ALPHA study, with no new safety concerns. Treatment with danicopan did not result in any serious adverse events. There were no reported deaths and meningococcal infections or discontinuations due to haemolysis.
Currently, applications seeking approval for danicopan for adults with PNH experiencing clinically significant EVH are under review with several regulatory bodies across the globe.
PNH is a rare fatal blood disorder that causes the destruction of red blood cells within blood vessels and white blood cell and platelet activation, which often leads to blood clots.
AstraZeneca PLC Price and Consensus
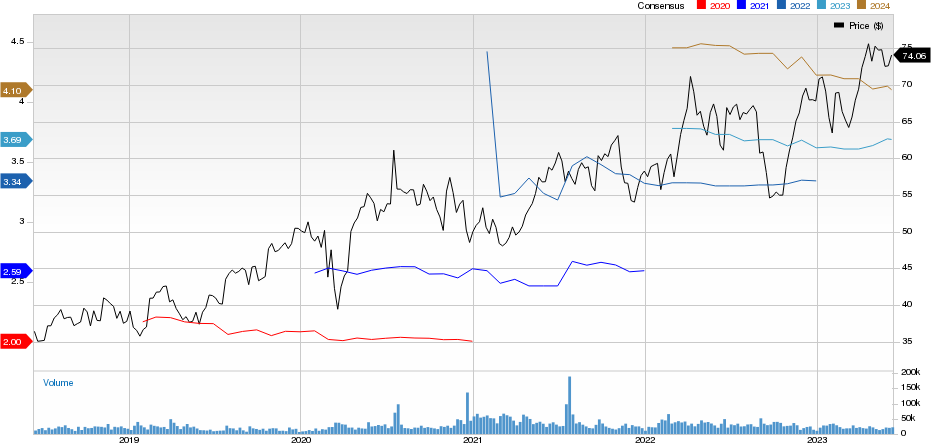
AstraZeneca PLC price-consensus-chart | AstraZeneca PLC Quote
Zacks Rank and Stocks to Consider
AstraZeneca currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the overall medical sector are Novartis NVS, Akero Therapeutics AKRO and Adaptimmune Therapeutics ADAP, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Novartis’ 2023 earnings per share has increased from $6.55 to $6.72. During the same period, the estimate for Novartis’ 2024 earnings has increased from $7.04 to $7.26. In the year so far, shares of Novartis have gained by 11.2%.
NVS beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 5.15%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $3.46 to $2.80. During the same period, the estimate for Akero Therapeutics’ 2024 loss per share has narrowed from $3.66 to $3.32. In the year so far, shares of Akero Therapeutics have fallen by 4.2%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
In the past 90 days, the Zacks Consensus Estimate for Adaptimmune Therapeutics’ 2023 loss per share has remained stable at 46 cents. During the same period, the estimate for Adaptimmune Therapeutics’ 2024 loss per share has narrowed from 74 cents to 56 cents. In the year so far, shares of Adaptimmune Therapeutics have fallen by 32.4%.
ADAP beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 36.89%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Adaptimmune Therapeutics PLC (ADAP) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
