AstraZeneca (AZN) Ultomiris Gets CHMP Nod for New Rare Disease
AstraZeneca AZN announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has given a positive opinion recommending approval of its long-acting C5 complement inhibitor, Ultomiris, for a new rare disease indication in the EU.
AstraZeneca is seeking approval for the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-aquaporin-4 (AQP4) antibody positive (Ab+).
If approved by the European Commission, Ultomiris would be the only drug approved for the treatment of AQP4 Ab+ NMOSD in the EU. Ultomiris is also under review in the United States for the treatment of NMOSD.
AstraZeneca’s stock has risen 4.1% in the past year compared with an increase of 4.8% for the industry.
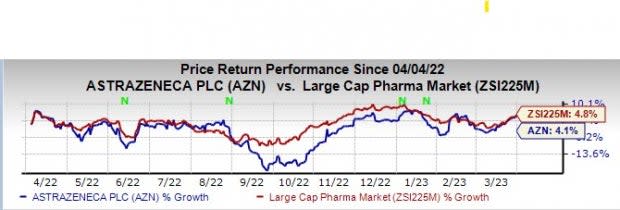
Image Source: Zacks Investment Research
The CHMP opinion was based on data from the CHAMPION-NMOSD phase III study evaluating Ultomiris in adults with AQP4 Ab+ NMOSD. Ultomiris met the primary endpoint of time to first on-trial relapse in the phase III study. Data from the study showed that Ultomiris achieved a statistically significant and clinically meaningful reduction in the risk of relapse in this patient population. No relapse was observed in 58 patients over a median treatment duration of 73 weeks in the study.
NMOSD is a rare autoimmune disease that affects the central nervous system (CNS), including the spine and optic nerves. Patients with this disease can experience unpredictable relapses, which can have long-term effects like vision loss, chronic pain and paralysis. Approximately 75% of patients with NMOSD are anti-AQP4 Ab+. Ultomiris reduced the risk of life-threatening relapses in patients with AQP4 Ab+ NMOSD in the CHAMPION-NMOSD study and can see significant demand if approved for the disease.
Ultomiris was added to AstraZeneca’s portfolio with the acquisition of Alexion and is already approved to treat paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome and generalized myasthenia gravis in the United States, EU and Japan.
Zacks Rank & Stocks to Consider
AstraZeneca has a Zacks Rank #3 (Hold).
Some better-ranked drugmakers/biotech companies are Novo Nordisk NVO Innoviva INVA and J&J JNJ. While Novo Nordisk has a Zacks Rank of 1 (Strong Buy), Innoviva and J&J have a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Estimates for Novo Nordisk’s 2023 earnings per share have increased from $4.18 to $4.48. Estimates for 2024 have jumped from $4.70 per share to $5.28 in the past 60 days. Novo Nordisk’s stock has surged 39.9% in the past year.
Novo Nordisk beat earnings expectations in three of the trailing four quarters. The company delivered a four-quarter earnings surprise of 3.00%, on average.
Estimates for J&J 2023 earnings per share have increased from $10.49 to $10.50 while that for 2024 have jumped from $10.91 per share to $10.94 in the past 60 days. J&J’s stock has declined 12.1% in the past year.
J&J beat earnings expectations in three of the trailing four quarters. The company delivered a four-quarter earnings surprise of 2.94%, on average.
Estimates for Innoviva’s 2023 earnings per share have increased from $1.04 to $1.37. Estimates for 2024 have jumped from 52 cents per share to $1.25 in the past 60 days. Innoviva’s stock has declined 43.3% in the past year.
Innoviva missed earnings expectations in three of the trailing four quarters. The company delivered a four-quarter negative earnings surprise of 50.78%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Innoviva, Inc. (INVA) : Free Stock Analysis Report
