AstraZeneca's (AZN) 3 Cancer Drugs Get CHMP Nod for Expanded Use
AstraZeneca AZN and partner Merck MRK announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency has given a positive opinion recommending the approval of their blockbuster PARP inhibitor Lynparza for the first-line treatment of metastatic castration-resistant prostate cancer (mCRPC) in Europe.
The regulatory application in Europe seeks approval for Lynparza in combination with J&J’s JNJ prostate cancer drug, Zytiga (abiraterone), and corticosteroid prednisone or prednisolone for mCRPC in patients for whom chemotherapy is not clinically indicated.
If approved by the European Commission, Lynparza with Zytiga will become the first PARP inhibitor to be approved in combination with a new hormonal agent for patients in the mCRPC setting in Europe.
The regulatory filing was based on data from the phase III study — PROpel. In the study, Lynparza plus J&J’s Zytiga and prednisone or prednisolone reduced the risk of disease progression or death by 34% versus Zytiga alone. Median radiographic progression-free survival (rPFS) was 24.8 months in the Lynparza plus Zytiga arm versus 16.6 months for Zytiga alone.
AstraZeneca stock has risen 9.7% this year so far compared with an increase of 2.9% for the industry.
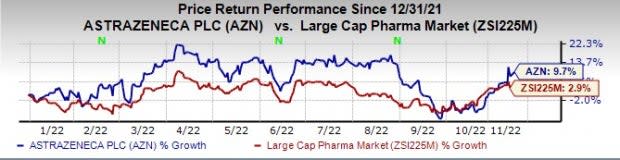
Image Source: Zacks Investment Research
A supplemental new drug application (sNDA) seeking approval of Lynparza with Zytiga and prednisone or prednisolone is under priority review in the United States, with an FDA decision expected in the fourth quarter of 2022.
At present, for prostate cancer, Lynparza is approved for BRCA-mutated mCRPC indication in several countries, including the United States and European Union, based on data from the PROfound phase III study.
AstraZeneca markets Lynparza in partnership with Merck. The profit-sharing deal between AstraZeneca and Merck was inked in 2017. In addition to Lynparza, the deal included Koselugo.
AstraZeneca & Merck’s Lynparza is approved for four cancer types, namely ovarian, breast, prostate and pancreatic. Lynparza is also being evaluated in an earlier-line setting for the approved cancer indications as well as some other cancer types.
The CHMP also recommended approving AstraZeneca and partner Daiichi Sankyo’s Enhertu for patients with previously treated HER2-positive advanced gastric cancer for patients who have received a prior trastuzumab-based regimen. The approval for this expanded use of Enhertu was based on data from the DESTINY-Gastric02 and the DESTINY-Gastric01 phase III studies
In the United States, Enhertu was approved for advanced or metastatic HER2-positive gastric cancer in January 2021. In the United States, Enhertu is also approved for previously treated HER2-mutant metastatic non-small cell lung cancer and metastatic HER2-positive breast cancers.
In Europe, Enhertu is presently approved for metastatic HER2-positive breast cancer in patients who have received two or more prior anti-HER2-based regimens.
The CHMP also recommended the approval of Imfinzi plus chemotherapy for the first-line treatment of unresectable or metastatic biliary tract cancer (BTC) based on the TOPAZ-1 phase III study. Updated survival data from the study showed that Imfinzi plus chemotherapy led to a 24% reduction in the risk of death versus chemotherapy alone. BTC is a group of rare and aggressive cancers that occur in the bile ducts and gallbladder.
In the United States, Imfinzi plus chemotherapy was approved for treating locally advanced or metastatic BTC in September.
Zacks Rank & Stock to Consider
AstraZeneca has a Zacks Rank #3 (Hold). A better-ranked biotech is Vertex Pharmaceuticals VRTX, which has a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Vertex Pharmaceuticals’ stock has risen 39.3% this year. Estimates for Vertex’s 2022 earnings have gone up from $14.21 to $14.61 per share, while those for 2023 have increased from $15.10 to $15.60 per share over the past 30 days.
Vertex has a four-quarter earnings surprise of 3.16%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
