AstraZeneca's (AZN) Imfinzi Combo Meets Liver Cancer Study Goal
AstraZeneca AZN announced positive results from the ongoing phase III EMERALD-I study, which is evaluating Imfinzi (durvalumab) as a combination therapy in hepatocellular carcinoma (HCC), the most common form of liver cancer.
The EMERALD-1 study is evaluating the combination of Imfinzi combined with bevacizumab and transarterial chemoembolization (TACE), compared with TACE alone, for the treatment of unresectable HCC in patients eligible for embolization.
The study achieved its primary endpoint of statistically significant and clinically meaningful improvement in progression-free survival (PFS). Data from the study also showed that treatment with the Imfinzi combination reduced the risk of disease progression or death in HCC patients by 23% when compared to patients treated with TACE alone.
The current standard of care for unresectable HCC patients, embolization, involves blocking the blood supply to the tumor and delivering chemotherapy or radiation therapy directly to the liver. Though AstraZeneca estimates that around 20-30% of HCC patients are eligible for embolization, the rate of disease progression with the disease remains high.
The median PFS was 15 months for patients treated with the Imfinzi combination compared to 8.2 months for those who received TACE alone. The study also achieved clinical benefit in the secondary endpoint of time to progression (TTP) as patients treated with the Imfinzi combination achieved a median TTP of 22 months, compared to 10 months in those who only received TACE.
Despite the above results, treatment with the Imfinzi combination also led to an increase in the rate of grade 3 or 4 treatment-related adverse events. Per management, 45.5% of patients who received the Imfinzi combination experienced adverse events compared to 23% of patients treated with TACE.
Shares of AstraZeneca have lost 1.6% year to date against the industry’s 16.7% rise.
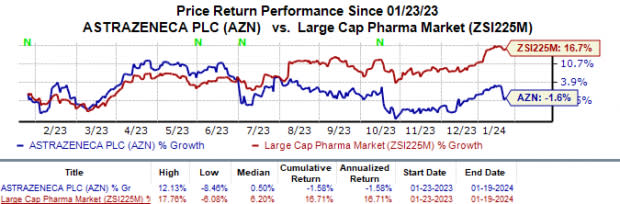
Image Source: Zacks Investment Research
AstraZeneca is currently discussing the above results with regulatory authorities. It plans to file the same after obtaining the final results on the key secondary endpoint of overall survival (OS).
If approved, this will be the second approval for Imfinzi in the HCC indication. The drug is already authorized in combination with Imjudo to treat adult patients with unresectable HCC. Apart from HCC, Imfinzi is approved for multiple cancer indications, including three in lung cancer and one in biliary tract cancer.
AstraZeneca is focused on strengthening its oncology business. In the first nine months of 2023, AZN generated $13.5 billion worth of total revenues from its Oncology business, reflecting a 20% year-over-year rise in the constant exchange rate, driven by a solid performance of newer medicines, such as Tagrisso, Lynparza, Imfinzi and Calquence. Management is working to further strengthen this portfolio through label expansions and advancing oncology pipeline candidates. The company intends to develop a treatment for every form of cancer.
In a separate press release, AstraZeneca also announced that its C5 inhibitor danicopan received its first regulatory approval in Japan to treat adults with paroxysmal nocturnal haemoglobinuria (PNH). The therapy will be marketed under the trade name Voydeya. Regulatory submissions for the drug are currently under review in multiple jurisdictions.
AstraZeneca PLC Price
AstraZeneca PLC price | AstraZeneca PLC Quote
Zacks Rank & Key Picks
AstraZeneca currently carries a Zacks Rank #3 (Hold).Some better-ranked stocks in the overall healthcare sector include CytomX Therapeutics CTMX, Novo Nordisk NVO and Sarepta Therapeutics SRPT. While CytomX and Novo Nordisk sport a Zacks Rank #1 (Strong Buy), Sarepta carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for CytomX Therapeutics for 2023 have swung from a loss of 10 cents per share to earnings of 2 cents. During the same period, estimates for 2024 have narrowed from a loss of 22 cents to a loss of 6 cents. Shares of CytomX have lost 40.3% in the past year.
CytomX Therapeutics’ earnings beat estimates in three of the last four quarters while missing the estimates on one occasion. On average, the company witnessed an average surprise of 45.44%. In the previous reported quarter, CytomX Therapeutics’ earnings beat estimates by 123.53%.
In the past 60 days, estimates for Novo Nordisk’s 2023 earnings per share have increased from $2.62 to $2.67. During the same period, the earnings estimates for 2024 have risen from $3.15 to $3.29. Shares of NVO have surged 51.6% in the past year.
Novo Nordisk’s earnings beat estimates in two of the last four quarters while meeting the mark on one occasion and missing the estimates on another. On average, the company witnessed an average surprise of 0.58%. In the last reported quarter, Novo Nordisk’s earnings beat estimates by 5.80%.
In the past 60 days, Sarepta’s loss estimates for 2023 have improved from a loss of $6.90 per share to $6.57. During the same period, earnings estimates for 2024 have risen from 98 cents to $2.14. Sarepta’s shares have lost 9.9% in the past year.
Sarepta’s earnings beat estimates in each of the last four quarters, delivering an average surprise of 48.67%. In the last reported quarter, Sarepta’s earnings beat estimates by 72.29%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
CytomX Therapeutics, Inc. (CTMX) : Free Stock Analysis Report
