AstraZeneca's (AZN) Lynparza Gets Expanded Use Nod in Japan
AstraZeneca AZN and partner Merck MRK announced that its drug Lynparza is approved for expanded use in prostate cancer in Japan. The approval in Japan granted Lynparza (olaparib), in combination with J&J’s Zytiga (abiraterone) and prednisolone, to treat adult patients with BRCA-mutated (BRCAm) castration-resistant prostate cancer with distant metastasis (mCRPC).
The approval by the Japanese regulatory body was based on encouraging and clinically meaningful results from a subgroup analysis of the PROpel phase III study. Per the data readout, the combination regimen of Lynparza and Zytiga reduced the risk of disease progression or death by 77% compared with Zytiga alone, portraying a hazard ratio of 0.23. The combo drug also reduced the risk of death by 61% compared with BRCAm mCRPC patients who only received Zytiga.
Lynparza is already approved in the EU and United States for similar expanded use. Currently, Lynparza is approved in Japan as a monotherapy for patients with BRCAm mCRPC based on the results of the Profound Study.
Year to date, shares of AstraZeneca have gained 0.1% compared with the industry’s 8.1% rise.
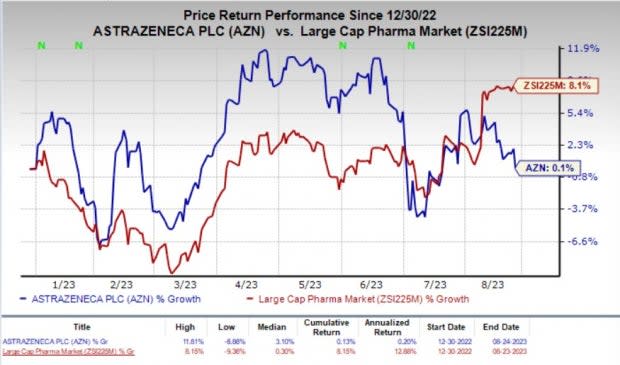
Image Source: Zacks Investment Research
Per AZN, prostate cancer is the most common cancer in men in Japan with a high mortality rate. Current treatment options in the region do not offer a satisfactory prognosis for mCRPC, especially for those patients whose cancer progresses following initial treatment. Thus, there is a high unmet medical need in this indication. Both AstraZeneca and Merck’s management believe that the approval of the Lynparza/abiraterone combo will provide the patients with a novel treatment option and has the potential to become a new standard-of-care in the treatment of BRCAm mCRPC.
Lynparza is currently approved in Japan and several other countries, including the EU and United States, as a monotherapy or in combination with other drugs to treat certain patients with four cancer types, such as ovarian, breast, prostate and pancreatic.
Lynparza is being jointly developed and commercialized by AstraZeneca and Merck. Per the press release, the drug has been used to treat more than 75,000 patients worldwide. AZN and MRK are evaluating Lynparza in earlier line setting for the approved cancer indications as well as some other cancer types. In a separate press release, AstraZeneca also announced that the Japanese regulatory authority has also approved its rare disease drug Soliris in Japan to treat generalized myasthenia gravis (gMG) in pediatric patients, who are anti-acetylcholine receptor antibody-positive (AChR Ab+). Soliris is already approved to treat adult patients with gMG in Japan.
Post approval from the Japanese regulatory body, Soliris became the first and only targeted therapy approved for the treatment of children and adolescents with gMG in Japan. The label extension of the drug is based on clinically meaningful improvement in the primary endpoint of the phase III study of Soliris in pediatric patients with refractory gMG.
Soliris is also currently approved in the EU for expanded use in refractory gMG in children and adolescents aged six to 17 years, who are AChR Ab+. Moreover, planned approvals in other countries for the same indication are either ongoing or scheduled ahead.
AstraZeneca PLC Price and Consensus
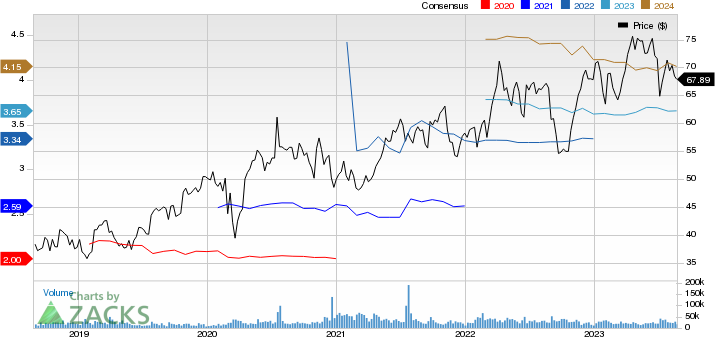
AstraZeneca PLC price-consensus-chart | AstraZeneca PLC Quote
Zacks Rank and Stock to Consider
AstraZeneca currently has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the pharma/biotech sector are J&J JNJ and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 (Buy) at present.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for J&J’s 2023 earnings per share has increased from $10.73 to $10.75. During the same period, the estimate for JNJ’s 2024 earnings per share has increased from $11.28 to $11.30. Year to date, shares of JNJ have lost 6.6%.
JNJ beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 5.58%.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has gone up from 62 cents to 78 cents. The estimate for Corcept’s 2024 earnings per share has also improved from 61 cents to 83 cents. Year to date, shares of CORT have climbed 56.4%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
