AstraZeneca's (AZN) sBLA for Enhertu Gets FDA's Priority Tag
AstraZeneca AZN announced that the FDA has granted priority review to a supplemental biologics license application (sBLA) seeking approval for the expanded use of Enhertu (trastuzumab deruxtecan) to treat unresectable or metastatic HER2-positive solid tumors in heavily-pretreated patients. A filing designated as a priority review reduces the review period by four months. A final decision is expected during the second quarter of 2024.
Enhertu, a specifically engineered HER2-directed antibody-drug conjugate (ADC), is jointly developed and commercialized by AstraZeneca and Daiichi Sankyo. It is currently approved for advanced or metastatic HER2-positive gastric cancer, previously treated HER2-mutant metastatic non-small cell lung cancer and metastatic HER2-positive breast cancers.
Per the press release, the sBLA is being reviewed under the Real-Time Oncology Review (RTOR) programand Project Orbis, the FDA’s two new initiatives focused on bringing safe and effective cancer treatments to patients as early as possible.
The unique nature of the RTOR program allows the FDA to review the components of an application before its complete submission. On the other hand, Project Orbis provides a framework for concurrent submission and review of oncology medicines among participating international partners.
In the past year, shares of AstraZeneca gained 2.8% compared with the industry’s 18.8% rise.
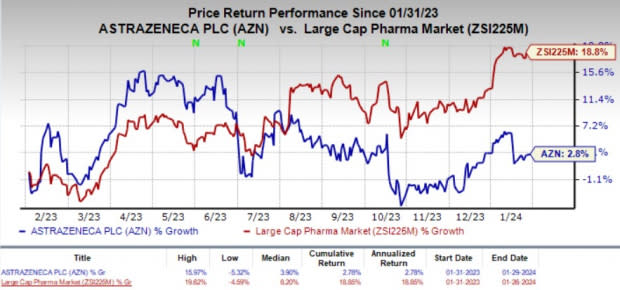
Image Source: Zacks Investment Research
The sBLA is based on positive results from the ongoing phase II DESTINY-PanTumor02 study of Enhertu for solid tumor indications. Data from the study showed that treatment with Enhertu resulted in clinically meaningful and durable responses leading to a clinically meaningful survival benefit in previously treated patients across HER2-expressing metastatic solid tumors, including biliary tract, bladder, cervical, endometrial, ovarian cancers and other tumors.
The sBLA submission also included data from other supporting studies of Enhertuin patients with HER2-positive IHC3+ tumors, including DESTINY-Lung01 (lung cancer study) and DESTINY-CRC02 (colorectal cancer study).
Subject to approval, Enhertu will become the first HER2-directed therapy and ADC with a tumor-agnostic indication.
AstraZeneca further stated that the safety profile of Enhertu was found to be consistent with previous studies of the drug and no new safety concerns were identified.
In August 2023, Enhertu received the FDA’s Breakthrough Therapy designation in the United States for the treatment of metastatic HER2-positive solid tumors.
The safety and efficacy of Enhertu monotherapy are currently being evaluated in a comprehensive clinical development program across multiple HER2-targetable cancers. Studies evaluating the drug in combination with other anticancer treatments, such as immunotherapy, are also underway.
AstraZeneca PLC Price and Consensus
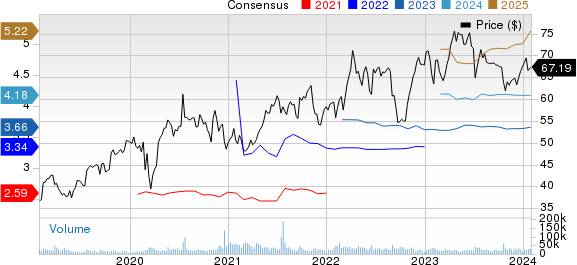
AstraZeneca PLC price-consensus-chart | AstraZeneca PLC Quote
Zacks Rank and Stocks to Consider
AstraZeneca currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry worth mentioning are Puma Biotechnology, Inc. PBYI, CytomX Therapeutics CTMX and Journey Medical DERM, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has increased from 72 cents to 73 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has increased from 64 cents to 69 cents. Over the past year, shares of PBYI have gained 15.8%.
PBYI beat estimates in three of the last four quarters while missing on one occasion, delivering a four-quarter average earnings surprise of 76.55%.
In the past 30 days, the Zacks Consensus Estimate for CytomX’s 2023 EPS has remained constant at 2 cents. The consensus estimate for CytomX’s 2024 loss per share is currently pegged at 6 cents. Over the past year, shares of CTMX have plunged 40.4%.
CTMX beat estimates in three of the trailing four quarters and missed the mark on one occasion, delivering an average earnings surprise of 45.44%.
In the past 30 days, the Zacks Consensus Estimate for Journey Medical’s 2023 loss per share has remained constant at 16 cents. During the same period, the consensus estimate for Journey Medical’s 2024 loss per share has remained constant at 69 cents. Over the past year, shares of DERM have skyrocketed 122.7%.
DERM beat estimates in one of the trailing four quarters and missed on the other three occasions, delivering an average earnings surprise of 118.25%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Journey Medical Corporation (DERM) : Free Stock Analysis Report
CytomX Therapeutics, Inc. (CTMX) : Free Stock Analysis Report
