Astria (ATXS) Rises 5% on Fast Track Tag for Angioedema Drug
Astria Therapeutics, Inc. ATXS announced that the FDA has granted fast track designation to its lead developmental candidate, STAR-0215, for the treatment of hereditary angioedema (HAE). Notably, HAE is characterized as a rare genetic disorder that causes severe unpredictable attacks of swelling throughout the body.
STAR-0215 is Astria’s monoclonal antibody, plasma kallikrein inhibitor, which is currently being evaluated in a phase Ib/II ALPHA-STAR study in HAE patients. Per the company, STAR-0215 has the potential to become the first-choice preventative treatment for people with HAE dosed once every three or six months. Treatment with STAR-0215 could benefit patients by improving their quality of life, thereby catering to a significant unmet medical need.
The stock of the company increased about 5% on Thursday in response to the encouraging regulatory update. Year to date, shares of Astria have plunged 33.8% compared with the industry’s 8.6% decline.
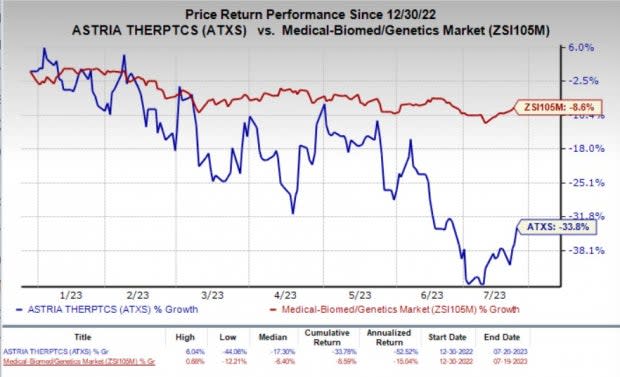
Image Source: Zacks Investment Research
The FDA’s fast track designations intend to expedite development and review timelines when preliminary nonclinical and clinical evidence indicates the drug may demonstrate substantial improvement over available therapies to address unmet medical needs for serious or life-threatening conditions. The fast track tag enables close communication between the FDA and sponsor to improve the efficiency of product development to get new therapeutics to patients faster.
Usually, companies, that receive the FDA’s fast track designation for a product candidate, can submit new drug application for such candidate on a rolling basis, which grants a faster review process of the application by the regulatory body. Additionally, drugs that receive fast track designation may be eligible for a priority review if certain criteria are met.
ATXS announced initiating the phase Ib/II ALPHA-STAR in February 2023. The study is enrolling patients with HAE types I and II. The early-mid-stage study will evaluate the safety and tolerability, changes in HAE attack rate, pharmacokinetics, pharmacodynamics and quality-of-life assessments, upon treatment with STAR-0215. Proof-of-concept results from the study are expected in mid-2024.
Per Astria, preliminary results from the phase Ia study in healthy subjects were coherent with STAR-0215’s target profile of being a long-acting preventative therapy, best-in-class pharmacokinetics profile and dosing once every three months or less frequently. Based on these encouraging results, the company plans to evaluate the potential for six-month administration of STAR-0215 in additional cohorts in the phase Ia study.
Preliminary data from the phase Ia additional cohorts are expected in the fourth quarter of 2023.
Astria Therapeutics, Inc. Price and Consensus
Astria Therapeutics, Inc. price-consensus-chart | Astria Therapeutics, Inc. Quote
Zacks Rank and Stocks to Consider
Astria currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same industry are ADC Therapeutics ADCT, Acadia Pharmaceuticals ACAD and Akoya Biosciences AKYA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for ADC Therapeutics’ 2023 loss per share has widened from $2.58 to $2.61. During the same period, the estimate for ADC Therapeutics’ 2024 loss per share narrowed from $2.72 to $2.45. In the past week, shares of ADCT have lost 36.7%.
ADCT beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 10.70%.
In the past 90 days, the Zacks Consensus Estimate for Acadia Pharmaceuticals’ 2023 loss per share has narrowed from 58 cents to 31 cents. The estimate for Acadia Pharmaceuticals’ 2024 earnings per share is pegged at 47 cents. In the past week, shares of ACAD have risen 33.2%.
ACAD beat estimates in two of the trailing four quarters, missing the mark on other two occasions, delivering an average negative earnings surprise of 2.75%.
In the past 90 days, the Zacks Consensus Estimate for Akoya Biosciences’ 2023 loss per share has narrowed from $1.80 to $1.71. During the same period, the estimate for Akoya Biosciences’ 2024 loss per share narrowed from $1.57 to $1.33. In the past week, shares of AKYA have lost 1.1%.
AKYA missed estimates in each of the trailing four quarters, delivering an average negative earnings surprise of 21.05%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ADC Therapeutics SA (ADCT) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
Akoya Biosciences, Inc. (AKYA) : Free Stock Analysis Report
Astria Therapeutics, Inc. (ATXS) : Free Stock Analysis Report
