Axsome (AXSM) Q3 Loss Widens, Revenues Surpass Estimates
Axsome Therapeutics, Inc. AXSM incurred an adjusted loss $1.32 per share in the third quarter of 2023, wider than the Zacks Consensus Estimate of a loss of $1.24. AXSM had reported a loss of $1.07 per share in the year-ago period.
However, the company’s $57.8 million revenues beat the Zacks Consensus Estimate of $55 million. AXSM had recorded revenues of $16.8 million in the year-ago period.
Revenues mainly benefited from the strong sales uptake of its two marketed products Auvelity (AXS-05) for major depressive disorder and Sunosi (solriamfetol) for narcolepsy.
Shares of AXSM were down 4.2% on Monday following the announcement of the news. The stock has lost 15.5% in the year-to-date period compared with the industry’s 21% decline.
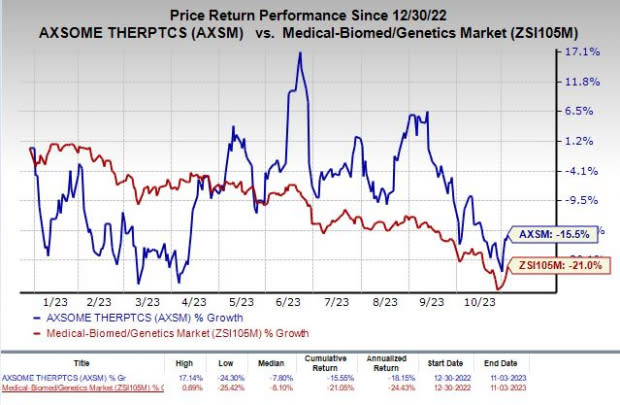
Image Source: Zacks Investment Research
Quarter in Detail
Total revenues consist of product revenues from Sunosi, Auvelity and royalty revenues.
Net product revenues were $57.1 million in the quarter compared with $16.8 million in the year-ago period. The figure beat our model estimate of $52.5 million.
Auvelity recorded sales of $37.7 million, up 36% from the previous quarter’s level, due to the products’ timely launch. There were no Auvelity sales in the comparable period of 2022. Sales of the drug beat our model estimate of $32.8 million.
Per the company, 69,000 prescriptions were recorded for Auvelity in the reported quarter, reflecting a sequential increase of 30%.
Axsome’s first drug, Auvelity, was approved for major depressive disorder by the FDA in August 2022 and was subsequently launched in October 2022.
Sunosi’s net product sales were $20.1 million, up 20% from the year-ago quarter’s level. Total prescriptions for Sunosi in the United States grew 16% year over year and 5% sequentially.
Axsome acquired the U.S. rights to Sunosi, a commercialized drug targeting narcolepsy, from Jazz Pharmaceuticals JAZZ in May 2022. It began selling Sunosi in the U.S. market in May 2022 and certain international markets in November 2022.
Jazz received approval for Sunosi as a treatment for narcolepsy in 2019.
In February 2023, Axsome out-licensed its ex-U.S. marketing rights of Sunosi to Pharmanovia. JAZZ is entitled to receive high single-digit royalty on net sales of Sunosi in the United States.
Royalty revenues totaled $0.7 million for the quarter, reflecting royalties Sunosi sales.
Research and development expenses (including stock-based compensation) amounted to $28.8 million, up almost 93.2% from the year-ago quarter’s level. The increase was due to higher costs associated with clinical studies, especially the label expansion study of Sunosi, as well as higher personnel and post-marketing commitments for Sunosi and Auvelity.
Selling, general and administrative expenses (including stock-based compensation) totaled $83.2 million, up almost 103.4% year over year. The significant increase was due to higher commercial activities for Sunosi and Auvelity.
As of Sep 30, 2023, Axsome had cash and cash equivalents worth $416.6 million compared with $437.1 million as of Jun 30, 2023.
2023 Guidance
Management believes that its cash balance of $416.6 million (as of September 2023-end) is enough to fund future operations into cash flow positivity.
Pipeline Updates
Axsome is evaluating Auvelity in several label expansion studies that are underway for the treatment of other central nervous system disorders. The phase III ADVANCE-2 study is evaluating the safety and efficacy of Auvelity for treating agitation associated with Alzheimer’s Disease. Enrollment in the study is expected to be completed in the first half of 2024.
The company also plans to start a pivotal phase II/III study of Auvelity for smoking cessation in 2024.
Axsome’s key pipeline candidates, including AXS-07, AXS-12 and AXS-14, target multiple central nervous system indications.
The lead candidate, AXS-07, is being developed for the acute treatment of migraine. In April 2022, Axsome received a complete response letter for a new drug application (NDA) seeking approval for AXS-07 for the acute treatment of the disease.
Axsome plans to resubmit its NDA in the first half of 2024. The FDA has not requested any additional safety or efficacy data for the candidate in light of the NDA resubmission.
AXS-12 is being evaluated in the phase III SYMPHONY study for the treatment of narcolepsy, with top-line data from the same expected in the first quarter of 2024.
AXSM plans to submit an NDA to seek approval of AXS-14 for the treatment of fibromyalgia in the first quarter of 2024 to the FDA.
The company is investigating Sunosi in a label expansion study – the phase III FOCUS study. The study evaluates the efficacy and safety of Sunosi for the treatment of attention deficit hyperactivity disorder. Top-line data from the study is expected by the second half of 2024.
Axsome Therapeutics, Inc. Price, Consensus and EPS Surprise
Axsome Therapeutics, Inc. price-consensus-eps-surprise-chart | Axsome Therapeutics, Inc. Quote
Zacks Rank & Stocks to Consider
Axsome currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Dynavax Technologies Corporation DVAX and MEI Pharma, Inc. MEIP. Each stock presently sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Dynavax Technologies’ 2023 loss per share have narrowed from 24 cents to 22 cents. Meanwhile, during the same period, earnings per share estimates for 2024 have improved from 2 cents to 8 cents. Year to date, shares of DVAX have rallied 33.6%.
Earnings of Dynavax Technologies beat estimates in two of the last four quarters while missing the same on the remaining two occasions. DVAX delivered a four-quarter average earnings surprise of 25.78%.
In the past 60 days, estimates for MEI Pharma’s 2023 loss per share have improved from $6.54 to $4.89. During the same period, loss per share estimates for 2024 have narrowed from $5.14 to $4.02. Year to date, shares of MEIP have rallied 46.1%.
Earnings of MEI Pharma beat estimates in three of the trailing four quarters and met the same on the other occasion. On average, MEIP delivered a four-quarter earnings surprise of 53.58%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Jazz Pharmaceuticals PLC (JAZZ) : Free Stock Analysis Report
MEI Pharma, Inc. (MEIP) : Free Stock Analysis Report
Axsome Therapeutics, Inc. (AXSM) : Free Stock Analysis Report
