Bausch Health (BHC) Q3 Earnings & Sales Beat, '23 View Updated
Bausch Health Companies Inc.'s BHC third-quarter results were better than expected. The company’s adjusted earnings per share of $1.03 beat the Zacks Consensus Estimate of 92 cents and increased from 76 cents reported in the year-ago quarter.
Total revenues of $2.24 billion were up 9% year over year. Revenues beat the Zacks Consensus Estimate of $2.15 billion.
Revenues in the quarter were affected by the unfavorable impact of foreign exchange of $6 million and the impact of divestitures and discontinuations of $19 million. Revenues were up 9% on an organic basis year over year.
The stock has gained 16.9% in the year so far compared with the industry’s growth of 15.7%.
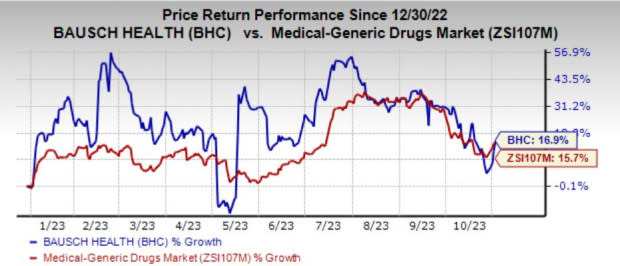
Image Source: Zacks Investment Research
Quarter in Detail
The company reports revenues through five segments – Salix, International, Diversified Products, Solta Medical and Bausch + Lomb.
Salix revenues came in at $614 million, up 13%, driven by growth in Xifaxan, Relistor (up 28%) and Trulance (up 10%). Xifaxan revenues grew by 13% in the quarter, driven by a year-over-year increase in wholesale channel inventory. Salix revenues beat the Zacks Consensus Estimate of $585 million and our model estimate of $599.4 million.
International revenues in the quarter were $275 million, up 10%, beating the Zacks Consensus Estimate of $262 million and our model estimate of $260 million. Revenues increased organically by 4%, driven by strong performances in Latin America and Poland, after excluding the unfavorable impact of foreign exchange.
Diversified Products revenues were $259 million, up 9% from the year-ago quarter due to increases in sales in Generics and Neurology. Dentistry experienced low-single-digit declines after two quarters of growth. Revenues beat the Zacks Consensus Estimate of $231 million. Growth in the dermatology product Jublia is expected to continue to boost this segment.
Solta Medical reported revenues of $83 million, up 15% and beat the Zacks Consensus Estimate of $81 million and our model estimate of $63.3 million. Revenues were up 17% organically, primarily driven by strong results in Asia Pacific.
Revenues from Bausch + Lomb were $1 billion, up 7% year over year and beating the Zacks Consensus Estimate of $996 million. The reported figure also beat our model estimate of $979.3 million. Excluding the unfavorable impact of foreign exchange of $10 million, acquisitions of $15 million and divestitures and discontinuations of $3 million, Bausch + Lomb segment revenues increased organically by 7% year over year, driven by increases across all business units.
Bausch + Lomb launched its initial public offering and subsequently began trading under the ticker "BLCO" on May 6, 2022.
Pipeline Development
Bausch received FDA approval of IDP-126, under the brand name Cabtreotm, the first and only FDA-approved fixed-dose, triple-combination topical treatment for acne.
The phase II study on amiselimod, a new oral S1P receptor modulator that targets the treatment of mild to moderate ulcerative colitis, completed enrollment in July. The induction portion of the study is expected to be completed in the fourth quarter.
The submission for next-generation Fraxel, a fractionated laser device for skin resurfacing, is planned for the first quarter of 2024 in the United States, with approval expected sometime in the first half of next year.
The company’s program for Clear and Brilliant Touch, a fractionated laser device for skin rejuvenation, is also advancing, with regulatory submissions planned for 2024 for Europe, Canada and Asia Pacific markets.
Other Updates
The FDA granted tentative approval to Norwich Pharmaceuticals Inc.’s abbreviated new drug application (“ANDA”) for Xifaxan (rifaximin) 550 mg in a letter dated Jun 2, 2023.
Xifaxan 550 mg tablets are indicated for reducing the risk of overt hepatic encephalopathy recurrence in adults and for treating irritable bowel syndrome with diarrhea in adults. However, the FDA confirmed in its letter that it cannot grant final approval until Oct 2, 2029, which is the date specified by the presiding judge in his final judgment in Salix Pharmaceuticals, LTD. et al. v. Norwich Pharmaceuticals, Inc. Norwich cannot launch its ANDA product until it receives final approval from the FDA. Norwich has sued the FDA in the United States District Court for the District of Columbia.
Norwich requested that the D.C. District Court direct the FDA to grant final approval of the ANDA, notwithstanding the Delaware Court's final judgment. The FDA opposed Norwich's action and BHC has also intervened in this lawsuit.
The consolidated appeals of the Delaware Court's ruling are ongoing at the Court of Appeals for the Federal District Circuit. BHC is awaiting a date for oral argument and expects a decision by the end of the first quarter of 2024.
Bausch Health Cos Inc. Price, Consensus and EPS Surprise
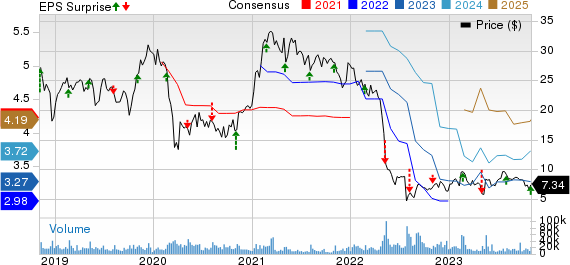
Bausch Health Cos Inc. price-consensus-eps-surprise-chart | Bausch Health Cos Inc. Quote
2023 Guidance Update
Revenues are projected in the range of $8.585-$8.710 billion (previous guidance: $8.45-$8.65 billion). Organic growth is now projected around 4% - 6% (earlier estimate: 2% -5%). Excluding Bausch + Lomb, revenues are projected to come to around $4.550 - $4.625 billion (previous guidance: $4.50- $4.65 billion). For the full year, Salix is expected to grow in the mid to high-single-digit range.
Gross margin is expected in the 80% range, in line with prior guidance.
Our Take
Bausch's third-quarter results were encouraging, as most of the business units posted growth. The Salix business maintains momentum.
However, the neurology and dermatology businesses continue to face long-term challenges that will impact the Diversified segment. Roughly 70% of revenues in this segment come from products that have lost exclusivity.
Zacks Rank and Stocks to Consider
Bausch currently carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the overall healthcare sector are Dynavax Technologies DVAX and Ligand Pharmaceuticals LGND, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Earnings estimates for Ligand Pharmaceuticals’ 2023 earnings per share have increased from $5.09 to $5.10 in the past thirty days. During the same period, earnings estimates for 2024 rose from $4.56 to $4.59. Shares of Ligand lost 19.3% in the year-to-date period.
Ligand beat earnings estimates in three of the last four quarters while missing the mark on one occasion. The company has delivered an earnings surprise of 52.47% on average.
Dynavax’s loss per share estimates for 2023 have narrowed from 23 cents to 22 cents for 2023 in the past 30 days. During the same period, earnings estimates for 2024 rose from 3 cents to 8 cents. Shares of DVAX have gained 22.5% in the year so far.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
Bausch Health Cos Inc. (BHC) : Free Stock Analysis Report
