BioMarin (BMRN) Q2 Earnings & Sales Top, Voxzogo Drives Sales
BioMarin Pharmaceutical BMRN reported second-quarter 2023 adjusted earnings per share of 54 cents, beating the Zacks Consensus Estimate of 47 cents.
The company had reported adjusted earnings of 41 cents per share in the year-ago quarter. Higher earnings were driven by an increase in revenues, which offset the impact of higher marketing costs for Roctavian’s launch.
Total revenues were $595.3 million in the reported quarter, up 12% year over year. This improvement was driven by the global uptake of Voxzogo and higher Palynziq revenues. The top line beat the Zacks Consensus Estimate of $591 million.
In the year-to-date period, the stock has fallen 15% compared with the industry’s decline of 11.2%.

Image Source: Zacks Investment Research
Quarter in Detail
Product revenues (including Aldurazyme) totaled $584.7 million, up 12.9% year over year. The same from BioMarin's marketed brands (excluding Aldurazyme) increased 13.3% year over year to $544.4 million on higher revenues from the new drug, Voxzogo. This offset lower sales from Kuvan and Naglazyme. Royalty and other revenues totaled $10 million, down 34.4% from the year-ago quarter's figure.
Vimizim sales rose 2% year over year to $177.4 million. Naglazyme sales were down 22% to $90.1 million due to the timing of orders in countries that place large government orders. Vimizim and Naglazyme sales fell short of our model estimates of $179 million and $118 million, respectively.
Brineura generated sales of $38.1 million, up 1% year over year. The figure missed our model estimates of $41.6 million for the quarter.
New drug Voxzogo, approved for achondroplasia, generated sales of $113.3 million compared with $87.8 million in the previous quarter. Higher sales of Voxzogo were driven by continued global market expansion and rapid patient uptake across all regions.
As of Jun 30, an estimated 2,000 children were being treated with Voxzogo from 36 active markets.
In the phenylketonuria franchise, Kuvan revenues declined 12% to $50.6 million due to generic competition. The drug lost U.S. market exclusivity in late 2020. BioMarin is now in the third year of U.S. generic competition for Kuvan and expects new generic competitors in Europe.
Palynziq injection sales grossed $74.9 million in the quarter, up 22% year over year, driven by patients initiating therapy, particularly in the United States.
Product revenues from Aldurazyme totaled $40.3 million, up 8% from that recorded in the prior-year quarter.
BioMarin has signed a collaboration agreement with Sanofi’s SNY subsidiary, Genzyme, for Aldurazyme. Sanofi, through Genzyme, is BMRN’s sole customer for Aldurazyme. The Sanofi subsidiary is also responsible for marketing and selling Aldurazyme to third parties.
2023 Sales Updated
Despite ongoing economic challenges like currency fluctuations, BioMarin slightly raised its previously issued Voxzogo sales guidance from a range of $380-$430 million to $400-$440 million. The guidance of the drug has been raised consecutively on the back of robust demand.
The company continues to expect Roctavian sales in the range of $50-$150 million. This is due to an extension in the PDUFA target action date.
Total revenues are projected around $2.4-$2.5 billion (unchanged).
Earnings per share are projected around $1.85-$2.10 (previous guidance: $1.80-$2.05).
BioMarin Pharmaceutical Inc. Price, Consensus and EPS Surprise
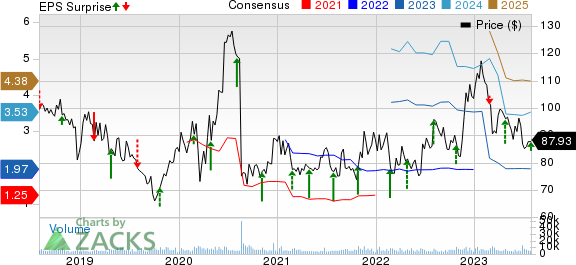
BioMarin Pharmaceutical Inc. price-consensus-eps-surprise-chart | BioMarin Pharmaceutical Inc. Quote
Pipeline Update
In June 2023, the FDA approved Roctavian, a gene therapy for the treatment of adults with severe hemophilia A (congenital factor VIII [FVIII] deficiency with FVIII activity < 1 IU/dL) without antibodies to adeno-associated virus serotype 5 detected by an FDA-approved test. The approval was granted based on data from the global phase III GENEr8-1 study. The gene therapy has already been conditionally approved by the European Commission in August 2022.
The commercial launch of the drug is underway.
BioMarin is looking to evaluate Voxzogo for a new potential second indication- hypochondroplasia, a condition characterized by impaired bone growth. Emerging data from an investigator-led phase II study and following receipt of feedback from FDA, BioMarin plans to begin the six-months observation arm of the study later this year, followed by the 52-week randomized, double-blind and placebo-controlled phase of the 80-participant clinical trial.
Zacks Rank & Stocks to Consider
Currently, BioMarin has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the overall healthcare sector are Alkermes ALKS and Dynavax Technologies DVAX, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Zacks Consensus Estimate for Alkermes’ earnings per share has moved up from 50 cents to $1.47 for 2023 and from $1.78 to $2.04 for 2024 in the past 60 days. The stock has risen 12% in the year-to-date period.
ALKS’ earnings beat estimates in all the trailing four quarters, the average surprise being 81.98%.
The loss per share estimate for DVAX has narrowed by 5 cents to 51 cents for 2023 in the past 30 days. Dynavax’ stock has risen 31.4% in the year-to-date period. DVAX’s earnings beat estimates in two of the trailing four quarters and missed in the remaining two, the average surprise being 65.18%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
BioMarin Pharmaceutical Inc. (BMRN) : Free Stock Analysis Report
Alkermes plc (ALKS) : Free Stock Analysis Report
