Biotech Stock Roundup: AMLX, ACAD Down on Study Data, MRNA, RGLS Gain on Updates & More
It was a busy week for the biotech sector with lots of study data readouts and regulatory approvals. Among these, Amylyx Pharmaceuticals AMLX and Acadia Pharmaceuticals ACAD were in the spotlight as shares of these companies were down on key study failures.
Recap of the Week’s Most Important Stories:
Amylyx Plummets on Study Failure: Amylyx Pharmaceuticals’ shares crashed after the company announced that the phase III PHOENIX study evaluating Relyvrio (AMX0035) for the treatment of amyotrophic lateral sclerosis (ALS) did not meet its primary or secondary endpoints.
The PHOENIX study failed to achieve statistical significance in the primary endpoint of the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) total score following 48 weeks of treatment with Relyvrio. The ALSFRS-R is a disease-specific severity score that reflects motor impairment and functional deterioration in ALS patients. The study also failed to achieve statistical significance in its secondary endpoints, which include quality of life patient-reported outcome assessments, overall survival and respiratory function.
Relyvrio obtained FDA approval in September 2022 to treat ALS in adults. The drug was also approved in June 2022 in Canada under the trade name Albrioza. Following the PHOENIX study’s failure, Amylyx will discuss the results with the regulatory authorities to decide whether to voluntarily withdraw Relyvrio from the U.S. and Canadian markets. Management has decided to pause all promotions for the drug for the time being.
Acadia Down on Study Failure: Acadia announced that the late-stage study evaluating pimavanserin for the treatment of negative symptoms of schizophrenia has failed. In the phase III ADVANCE-2 study, treatment with pimavanserin did not achieve statistical significance in demonstrating improvement over placebo on the study’s primary endpoint of change from baseline to week 26 on the Negative Symptom Assessment-16 (NSA-16) total score. The NSA-16 scale is a widely used metric to measure changes in the wide range of predominant negative symptoms that patients experience.
Consequently, Acadia has decided not to conduct any further clinical studies with pimavanserin. Shares were down on the news. However, the company will continue to analyze data from the schizophrenia study of pimavanserin.
Pimavanserin was, however, well-tolerated in the ADVANCE-2 study and had a safety profile consistent with the previous clinical studies. Pimavanserin is currently marketed in the United States as the first and only FDA-approved treatment for hallucinations and delusions associated with Parkinson’s disease psychosis under the brand name Nuplazid.
Regulus Gains on Study Data: Regulus Therapeutics RGLS announced positive top-line data from the second cohort of the ongoing phase Ib multiple-ascending dose (MAD) study on its lead product candidate RGLS8429 in autosomal dominant polycystic kidney disease (ADPKD). The company’s shares surged on the news.
The second cohort enrolled 14 participants who were randomized in a ratio of 3:1 to receive either 2 mg/kg of RGLS8429 or a placebo every other week for three months.
Data from the study showed strong evidence of the drug's effectiveness of a mechanistic dose response at a 2mg/kg dose based on urinary measurements of polycystins 1 and 2 (PC1 and PC2). The urinary measurements demonstrated greater biological activity of RGLS8429 at 2mg/kg compared to 1mg/kg and placebo. Treatment with the drug was well tolerated by the participants with no safety concerns.
Management also shared exploratory results from three patients with the highest increase in PC1 and PC2. These patients experienced reductions in height-adjusted total kidney volume (htTKV) greater than 4%, along with a decrease in total kidney cyst volume (TKCV). Based on the above results, management expanded the sample size of the fourth cohort of the MAD study from 14 to up to 30 patients. This cohort is evaluating a fixed dose of 300mg of RGLS8429 dosed every other week for three months. Screening is expected to start in the second quarter of 2024.
In addition, Regulus recently held a Type D meeting with the FDA to discuss the accelerated approval pathway. The meeting was constructive and confirmed the potential for an accelerated approval pathway based on a single-phase II study of RGLS8429 for the treatment of ADPKD.
Moderna Gains on Study Initiation: Moderna’s MRNA shares rose following details of the initiation of a phase II/III study on mRNA-4157/V940, its investigational individualized neoantigen therapy (INT), in patients with cutaneous squamous cell carcinoma (CSCC). The details of the initiation were shared by a government website. The phase II/III study (called INTerpath-007) will evaluate the safety and efficacy of mRNA-4157, combined with Merck’s blockbuster immuno-oncology drug Keytruda, as neoadjuvant and adjuvant therapy in patients with resectable locally advanced CSCC, when compared with standard of care (SoC) only. The study is expected to begin next month.
The primary endpoint of the INTerpath-007 study is event-free survival (EFS). The secondary endpoints include overall response rate (ORR), disease-free survival (DFS) and overall survival (OS).
The INTerpath-007 study will be Moderna/Merck’s third clinical study in the INTerpath program evaluating INT in multiple cancer indications.
Regeneron’s Praluent Label Extension: Regeneron REGN announced that the FDA extended the approval of the cholesterol drug Praluent’s (alirocumab) label. The regulatory body approved the drug as an adjunct to diet and other low-density lipoprotein cholesterol (LDL-C) lowering therapies to include pediatric patients aged eight and older with heterozygous familial hypercholesterolemia (HeFH).
The approval is based on a phase III, randomized multi-center trial evaluating pediatric patients aged 8 to 17 with HeFH, who had LDL-C levels of 130mg/dL or greater and were already being treated with lipid-lowering medications. Praluent is already indicated in the United States to reduce the risk of myocardial infarction, stroke and unstable angina requiring hospitalization in adults with established cardiovascular disease. The drug is indicated as an adjunct to diet, alone or in combination with other low-density LDL-C lowering therapies in adults with primary hyperlipidemia, including HeFH to reduce LDL-C.
REGN currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Performance
The Nasdaq Biotechnology Index has gained 0.07% in the past five trading sessions. Among the biotech giants, Moderna’s shares have risen 8.57% during the period. Over the past six months, shares of GSK have surged 20.28%. (See the last biotech stock roundup here: Biotech Stock Roundup: MRNA’s Q4 Results, RAPT Down on Study Results & Other Updates)
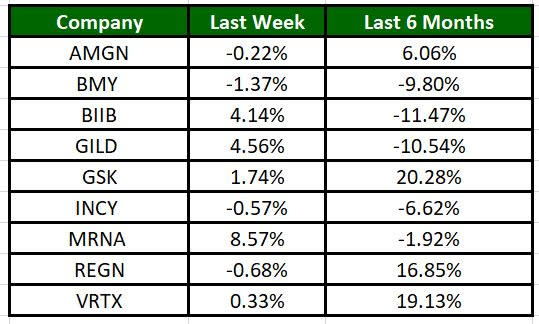
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Regulus Therapeutics Inc. (RGLS) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
Amylyx Pharmaceuticals, Inc. (AMLX) : Free Stock Analysis Report
