Biotech Stock Roundup: BMRN's Gene Therapy Gets Approval, SGTX Up on Buyout by LLY
It was a busy week for the biotech sector with many important regulatory and pipeline updates. Among these, BioMarin Pharmaceutical Inc.’s BMRN gene therapy approval grabbed the spotlight.
Recap of the Week’s Most Important Stories:
BioMarin’s Gene Therapy Approval: BioMarin won the FDA’s approval for gene therapy Roctavian (valoctocogene roxaparvovec-rvox). The therapy has been approved for the treatment of adults with severe hemophilia A (congenital factor VIII (FVIII) deficiency with FVIII activity < 1 IU/dL) without antibodies to adeno-associated virus serotype 5 (AAV5) detected by an FDA-approved test.
The gene therapy was granted conditional approval in the European Union for adults last August. The FDA approval is based on three-year follow-up safety and efficacy data from the phase III GENEr8-1 study. Study participants, who were administered the gene therapy, experienced a mean annualized bleeding rate (ABR) reduction of 52% compared with those who were administered the current standard-of-care (SOC) of treatment, i.e., a prophylaxis regimen with Factor VIII (FVIII).
Amneal Parkinson’s Drug Suffers a Setback: Amneal Pharmaceuticals, Inc. AMRX received a CRL from the FDA regarding the NDA for IPX203 for the treatment of Parkinson’s disease. Shares were down on the same. The CRL indicated that the data from pharmacokinetic studies suggest an adequate scientific bridge was established for the safety of one ingredient, levodopa (LD), but it was not adequately established for the other ingredient, carbidopa (CD). Consequently, the regulatory body requested additional information.
The letter did not identify any issues with respect to the efficacy or manufacturing of IPX203. Amneal will work closely with the FDA to address its comments and plans to meet with the agency to align on the best path forward.
IPX203 is a novel oral formulation of CD/LD extended-release capsules designed for the treatment of Parkinson’s disease. Amneal is developing IPX203 to provide a longer duration of therapeutic benefit than existing formulations with fewer doses. The NDA, based on the results from the late-stage RISE-PD clinical trial, was accepted by the FDA in November 2022.
Updates From Moderna: Moderna MRNA announced that it submitted marketing authorization applications for mRNA-1345, a vaccine for the prevention of RSV-associated lower respiratory tract disease (RSV-LRTD) and acute respiratory disease (ARD) in adults aged 60 years or older. Regulatory submissions have also begun in Switzerland and Australia. The company has also initiated the rolling submission process for a biologics license application (BLA) to the FDA for the licensure of the mRNA-based RSV vaccine.
The regulatory filings are based on positive data from an interim analysis of the pivotal phase III ConquerRSV study. Data from the study showed that participants who received mRNA-1345 achieved vaccine efficacy of 83.7% against RSV-LRTD, defined by two or more symptoms of the disease in older adults. The vaccine also achieved 82.4% efficacy against RSV-LRTD, defined by three or more symptoms of the disease. mRNA-1345 was well-tolerated and no safety concerns were identified.
Moderna also announced that it has submitted a regulatory application to the European Medicines Agency seeking approval for mRNA-1273.815, its updated COVID-19 vaccine targeting the XBB descendent lineage viruses. The regulatory filing is based on the preliminary data from preclinical studies that demonstrated the effectiveness of mRNA-1273.815 in generating an immune response against the current XBB variants of concern.
Sigilon Surges on LLY Acquisition: Shares of Sigilon Therapeutics, Inc. SGTX surged after its acquisition announcement by pharma giant Eli Lilly LLY. Per the terms, Lilly will acquire all outstanding shares of Sigilon for a purchase price of $14.92 per share in cash (an aggregate of approximately $34.6 million) payable at closing, plus one non-tradeable contingent value right (CVR) per share that entitles the holder to receive up to an additional $111.64 per share in cash, for a total potential consideration of up to $126.56 per share in cash without interest. This CVR, which is tied to clinical and regulatory milestones, brings the total value of the deal up to $309.6 million. In addition, the CVR holders would be entitled to receive contingent payments of $4.06 per share in cash upon first dosing of a specified product in the first human clinical study, $26.39 per share in cash in the first human clinical study for registration and $81.19 per share in cash upon receipt of the first regulatory approval of a specified product.
This acquisition deal is an extension of an agreement between the companies in 2018 to jointly develop encapsulated cell therapies for treating type 1 diabetes (T1D). Using these therapies, Lilly intends to develop novel cell therapies that free patients from constant disease management by sensing blood glucose levels, restoring insulin production and releasing it over the long term.
Sigilon currently has a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Performance
The Nasdaq Biotechnology Index has gained 0.12% in the past four trading sessions. Among the biotech giants, Incyte has gained 3.35% during the period. Over the past six months, shares of MRNA have lost 28.80%. (See the last biotech stock roundup here: Biotech Stock Roundup: SRPT's DMD Therapy Approval, ICPT's Setback & More Updates).
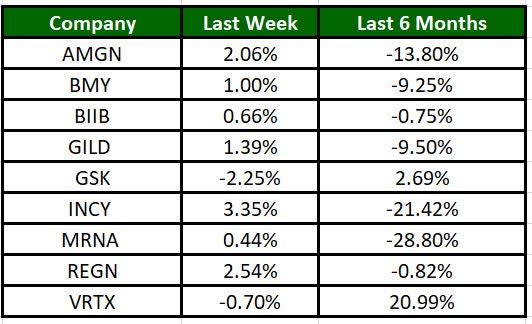
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for more pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
BioMarin Pharmaceutical Inc. (BMRN) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
AMNEAL PHARMACEUTICALS, INC. (AMRX) : Free Stock Analysis Report
Sigilon Therapeutics, Inc. (SGTX) : Free Stock Analysis Report
