Biotech Stock Roundup: EXEL Partners RCUS for Study, PHVS & EYPT Gain on Study Data
It was a busy week for the biotech sector, with numerous study readouts and other updates. Quite a few companies offered important updates on their key candidates. While nothing much came from biotech bigwigs, Exelixis EXEL announced a collaboration for its renal cell carcinoma study. Pharvaris PHVS and EyePoint Pharmaceuticals EYPT announced positive updates from studies.
Recap of the Week’s Most Important Stories:
Exelixis Collaborates With Arcus: Exelixis entered into a clinical collaboration with clinical-stage company Arcus Biosciences RCUS for its phase Ib/II study STELLAR-009. This study is evaluating zanzalintinib, a next-generation oral tyrosine kinase inhibitor, in combination with AB521 in patients with advanced solid tumors, including clear cell renal cell carcinoma (“ccRCC”).
While the study is being sponsored by Exelixis, Arcus is co-funding it and providing AB521 for use. ccRCC is the most common type of kidney cancer in adults. Patient enrollment in the STELLAR-009 study is expected to begin shortly. The dose-finding stage of the STELLAR-009 study will determine a recommended dose for zanzalintinib in combination with AB521 in patients with advanced solid tumors and patients with advanced ccRCC. The expansion cohorts will then evaluate the tolerability and activity of this combination in ccRCC. It will also assess the contribution of components, supported by activity data generated from monotherapy studies in ccRCC patients, to support full development.
Concurrently, Exelixis initiated a phase II/III STELLAR-305 study evaluating zanzalintinib in combination with Keytruda (pembrolizumab) compared with standalone Keytruda in patients with previously untreated PD-L1-positive recurrent or metastatic squamous cell carcinoma of the head and neck (“SCCHN”). This double-blind phase II/III study will enroll patients with PD-L1-positive recurrent or metastatic SCCHN that is incurable with local therapies. Enrolled patients will be equally randomized in two arms to receive zanzalintinib in combination with Keytruda or placebo in combination with Keytruda.
Pharvaris Surges on Study Data: Shares of clinical-stage company, Pharvaris, soared after the company announced that the mid-stage study, CHAPTER-1, met its primary endpoint. The double-blind, placebo-controlled phase II study is evaluating the efficacy as well as the safety and tolerability of deucrictibant for long-term prophylaxis against angioedema attacks in hereditary angioedema (HAE).
Results showed that 40 mg/day of orally administered deucrictibant significantly reduced the mean monthly attack rate by 84.5% compared to placebo, thereby meeting the primary endpoint. In the analysis of the secondary endpoints, deucrictibant demonstrated a clinically meaningful improvement in the severity of attacks and a decrease in the number of attacks treated with on-demand medication.
In June, Pharvaris announced the removal of the clinical hold of deucrictibant for the on-demand treatment of HAE in the United States. The company also completed the 26-week rodent toxicology study requested by the FDA and is preparing to submit the study results to the regulatory body by the end of the year.
BMY’s Opdivo’s Label Expansion: Bristol Myers BMY announced that the FDA has accepted the company’s supplemental biologics license application (sBLA) for Opdivo (nivolumab). The sBLA is seeking label expansion of Opdivo in combination with cisplatin-based chemotherapy as a first-line treatment for adult patients with unresectable or metastatic urothelial carcinoma.
The regulatory body granted the application Priority Review and assigned a target action date of Apr 5, 2024. The sBLA was based on the results from the phase III CheckMate -901 study, wherein the combination showed statistically significant and clinically meaningful survival benefits over standard-of-care gemcitabine plus cisplatin in the treatment of this patient population.
Bristol Myers also announced that its CAR T cell immunotherapy, Abecma, is now approved for use in earlier lines of therapy for patients with relapsed or refractory multiple myeloma in Japan. The company also announced a quarterly dividend of 60 cents per share, a 5.3% increase over last year’s quarterly rate of 57 cents.
BMY currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
EyePoint Surges on Wet AMD Data: EyePoint Pharmaceuticals surged after the company announced positive top-line results of its phase II study, EYP-1901. EYP-1901 is EyePoint’s novel sustained delivery maintenance treatment, which is being developed by combining vorolanib, a selective tyrosine kinase inhibitor, with the company’s proprietary bioerodible Durasert E technology. The phase II DAVIO 2 study enrolled 160 wet AMD patients who were previously treated with a standard-of-care anti-VEGF therapy. The study met its primary endpoint with both EYP-1901 doses (2mg and 3 mg), observing a statistical non-inferiority change in best corrected visual acuity compared with Eylea.
The mid-stage study also achieved key secondary endpoints with both EYP-1901 doses, which included a reduction in treatment burden of more than 80%, nearly two-thirds of eyes supplement-free up to six months and more than 80% receiving only zero or one supplement up to six months. Treatment with EYP-1901 demonstrated a favorable safety profile with no drug-related ocular or systemic serious adverse events.
Performance
The Nasdaq Biotechnology Index has gained 4.2% in the past five trading sessions. Among the biotech giants, Gilead has gained 5.49% during the period. Over the past six months, shares of Moderna have plunged 36.49%. (See the last biotech stock roundup here: Biotech Stock Roundup: RNA Up on BMY Deal Expansion, BIVI Down on Setback & More).
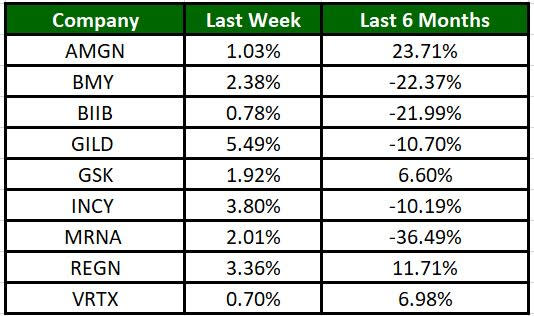
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for more pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
EYEPOINT PHARMACEUTICALS, INC. (EYPT) : Free Stock Analysis Report
Arcus Biosciences, Inc. (RCUS) : Free Stock Analysis Report
Pharvaris N.V. (PHVS) : Free Stock Analysis Report
