Biotech Stock Roundup: GERN, CRNX Gain on Updates, BMY's CAR T Cell Therapy News & More
It was a busy week for the biotech sector with lots of study data readouts and regulatory approvals. Among these, Geron Corporation GERN and Crinetics Pharmaceuticals CRNX gained on positive regulatory and study updates, respectively.
Recap of the Week’s Most Important Stories:
Updates From Bristol Myers: Bristol Myers BMY announced that the European Commission (EC) has approved a label expansion chimeric antigen receptor (CAR) T cell immunotherapy Abecma (idecabtagene vicleucel; ide-cel). The CAR T cell therapy is now approved in the European Union (EU) in earlier lines for triple-class exposed relapsed and refractory multiple myeloma. Abecma is already approved in the EU, Switzerland, Japan, the United Kingdom and Israel for adult patients with triple-class exposed relapsed or refractory multiple myeloma after three to four or more prior lines of therapy.
Earlier, the FDA Oncologic Drugs Advisory Committee (ODAC) voted in favor (8:3) of Abecma’s benefit/risk profile for patients with triple-class exposed relapsed and refractory multiple myeloma in earlier lines of therapy. The favorable voting was also based on results from the KarMMa-3 study, including the key secondary endpoint of overall survival.
The FDA also granted accelerated approval for BMY’s other CAR T cell therapy, Breyanzi (lisocabtagene maraleucel), for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma, who have received at least two prior lines of therapy, including a Bruton tyrosine kinase inhibitor and a B-cell lymphoma 2 inhibitor. Breyanzi is already approved in the United States, Japan and Europe for the second-line treatment of relapsed or refractory large B-cell lymphoma.
Geron Gains on Regulatory Update: Shares of GERN gained after the company announced that the ODAC voted in favor of the clinical benefit/risk profile of pipeline candidate, imetelstat, for the treatment of transfusion-dependent (TD) anemia in adult patients with low-to-intermediate-1 risk myelodysplastic syndromes (LR-MDS) who have not responded to or have lost response to or are ineligible for erythropoiesis-stimulating agents (ESAs). The ODAC voted 12:2 in favor of imetelstat’s benefits over risks.
The favorable decision by the ODAC was based on results from the phase III IMerge clinical trial. The primary endpoint of red blood cell transfusion independence (RBC-TI), for at least eight consecutive weeks, was significantly higher with imetelstat as compared to placebo. Additionally, 28% of imetelstat-treated patients experienced a statistically significant improvement in the key secondary endpoint of at least 24-week RBC-TI as compared to 3% on placebo. The median duration was 80 weeks for those achieving ≥24-week RBC-TI. Geron is currently gearing up for a prospective launch of the drug in the United States.
Crinetics Up on Study Success: Crinetics Pharmaceuticals reported positive top-line data from the second (PATHFNDR-2) of two late-stage studies evaluating the efficacy and safety of lead candidate, paltusotine, for the treatment of acromegaly. Shares of the company gained on the same.
The phase III PATHFNDR-2 study met its primary endpoint, with statistical significance, observing that 56% of patients treated with paltusotine achieved an insulin-like growth factor 1 (IGF-1) level ≤ 1.0 times the upper limit of normal (xULN) compared to those who received placebo (5%). Crinetics also reported that all secondary endpoints met statistical significance. Additionally, CRNX stated that the candidate was overall well tolerated with no serious adverse events in the PATHFNDR-2 study.
Crinetics is currently gearing up to submit a regulatory application to the FDA seeking approval of paltusotine for the acromegaly indication in the second half of 2024. The company aims to launch the product in the U.S. market in 2025.
Axsome Initiates Depression Study: Axsome Therapeutics, Inc. AXSM announced that the first patient has been dosed in the phase III PARADIGM study of solriamfetol, an investigational treatment for major depressive disorder (MDD). The double-blind and placebo-controlled phase III study will evaluate the safety and efficacy of solriamfetol (300 mg) in adult patients with MDD. The primary endpoint of the study is to see the change in the Montgomery Åsberg Depression Rating Scale. Solriamfetol is marketed in the United States under the trade name, Sunosi, for the treatment of narcolepsy.
Axsome acquired the U.S. rights to Sunosi from Jazz Pharmaceuticals in May 2022 and began selling the drug right after. The company also started selling the drug in certain international markets in November 2022.
PTCT Gains on Regulatory Updates: PTC Therapeutics, Inc. PTCT announced that it has submitted a biologics license application (BLA) for gene therapy Upstaza (eladocagene exuparvovec) to the FDA. The BLA is seeking approval of the therapy for the treatment of aromatic L-amino acid decarboxylase (AADC) deficiency, a fatal, rare genetic disorder that typically causes severe disability in babies.
Upstaza has received marketing authorization in Europe, Great Britain and Israel. It is a one-time gene replacement therapy indicated for the treatment of patients aged 18 months and older with a clinical, molecular and genetically confirmed diagnosis of AADC deficiency with a severe phenotype.
Concurrently, PTC Therapeutics announced plans to re-submit a new drug application (NDA) for Translarna (ataluren) for the treatment of nonsense mutation Duchenne muscular dystrophy (nmDMD), based on recent feedback from the FDA. The NDA resubmission is targeted by the middle of 2024. Translarna is a protein restoration therapy, designed to enable the formation of a functioning protein in patients with genetic disorders caused by a nonsense mutation.
PTCT also announced that the marketing authorization application for sepiapterin for the treatment of phenylketonuria remains on schedule for submission to the European Medicines Agency by the end of this month. Shares of the company gained on these updates.
PTCT currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Performance
The Nasdaq Biotechnology Index has lost 1.15% in the past five trading sessions. Among the biotech giants, Biogen’s shares have lost 3.71% during the same time frame. Over the past six months, shares of VRTX have surged 16.97%. (See the last biotech stock roundup here: Biotech Stock Roundup: AMLX, ACAD Down on Study Data, MRNA, RGLS Gain on Updates & More)
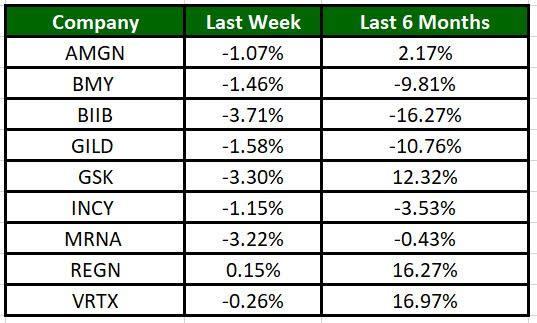
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Geron Corporation (GERN) : Free Stock Analysis Report
PTC Therapeutics, Inc. (PTCT) : Free Stock Analysis Report
Axsome Therapeutics, Inc. (AXSM) : Free Stock Analysis Report
Crinetics Pharmaceuticals, Inc. (CRNX) : Free Stock Analysis Report
