Biotech Stock Roundup: ICPT Up on Buyout Deal, IMVT & PLRX Gain on Study Data
It was a busy week for the biotech sector, with a lot of important regulatory and pipeline updates. Among these, Intercept Pharmaceuticals, Inc. ICPT surged on an acquisition deal with Alfasigma S.p.A and Immunovant IMVT soared on study results.
Recap of the Week’s Most Important Stories:
Intercept Soars on Acquisition News: Shares of Intercept soared after Italy-based pharmaceutical company Alfasigma announced an agreement to acquire it for $19.00 per share in cash. The purchase price of $19 represented a premium of 82% to Intercept’s closing stock price on Sep 25, 2023. Investors cheered the premium offer and shares surged on the same. The transaction is expected to close by the end of the year.
Intercept’s lead drug, Ocaliva (obeticholic acid or OCA), a farnesoid X receptor agonist, is approved in the United States and several other jurisdictions for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults with an inadequate response to UDCA or as monotherapy in adults intolerant to UDCA. Ocaliva is the only FDA-approved second-line therapy for PBC.
The acquisition news came in troubled times for Intercept, which earlier shelved plans for its nonalcoholic steatohepatitis treatment. The company also decided to cut one-third of its workforce to reduce operating expenses. Hence, the decision to merge with Alfasigma should help Intercept wade through these times better and market Ocaliva.
Immunovant Surges on Study Data: Shares of Immunovant, Inc. IMVT surged after the company announced positive initial results from both cohorts, the single-ascending dose (SAD) portion and the multiple-ascending dose (MAD) portion of its early-stage study of IMVT-1402. Subcutaneously administered (SC) IMVT-1402 demonstrated a consistent reduction in immunoglobulin G (IgG) with potency that was similar to or greater than that of batoclimab in the SAD portion of the study. Data showed there was no significant reduction from baseline in serum albumin or increase in lipoprotein cholesterol (LDL-C) observed at any timepoint measured. IMVT-1402 is being developed as a simple SC injection.
No decrease in serum albumin below baseline or increase in low-density LDL-C above baseline was observed after four weeks of dosing in the 300 mg MAD SC cohort. After four weekly 300 mg SC doses of IMVT-1402, the mean total IgG reduction from baseline in this MAD cohort was 63%.
Immunovant currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Pliant Up on Study Results: Shares of Pliant Therapeutics, Inc. PLRX gained earlier in the week after the company announced that its mid-stage study on lead pipeline candidate bexotegrast was successful. The phase IIa INTEGRIS-PSC study, a randomized, dose-ranging, double-blind, placebo-controlled trial, is evaluating bexotegrast at once-daily doses of 40 mg, 80 mg, 160 mg or placebo for 12 weeks in 85 patients with primary sclerosing cholangitis (PSC). The trial met its primary and secondary endpoints, demonstrating that the candidate was well tolerated over a 12-week treatment period and that its plasma concentrations increased with dosage.
Of the 64 patients treated with bexotegrast, 60 completed 12 weeks of treatment with no deaths or drug-related severe adverse events. The treatment-emergent adverse events that came up were mild or moderate in severity and consistent with PSC disease symptoms. Patients in the trial who had concomitant inflammatory bowel disease (IBD) saw no change in their IBD symptoms as measured by the partial Mayo Score while on treatment.
Bexotegrast was effective in reducing both Enhanced Liver Fibrosis scores and PRO-C3 levels at week 12 at all doses relative to placebo, as observed from the results of the three initial doses evaluated. Statistically significant differences were observed at the 160 mg dose relative to placebo at week 12. The enrolled patients also showed stabilization of liver chemistry, including a dose-dependent trend in the reduction of alkaline phosphatase levels relative to placebo. Preliminary magnetic resonance imaging results suggested improved hepatocyte function and bile flow with bexotegrast 160 mg.
Ionis Up on Study Results: Ionis Pharmaceuticals, Inc. IONS announced positive top-line results from the late-stage BALANCE study on its investigational candidate olezarsen in people with familial chylomicronemia syndrome (FCS). The candidate is an investigational LIgand Conjugated Antisense drug being evaluated for people at risk of disease due to elevated triglyceride (TG) levels, including those with FCS.
This phase III study achieved its primary endpoint of a statistically significant reduction in TG levels at six months with an 80mg dose of olezarsen. Additionally, olezarsen 80 mg showed a 100% reduction in acute pancreatitis events compared to placebo (0 events for olezarsen versus 11 events for placebo), a key secondary endpoint.
Data from the study demonstrated a dose-dependent effect following treatment with olezarsen. The study also evaluated a 50mg dose of the drug. Though treatment with this dosage level substantially reduced pancreatitis, it did not achieve the BALANCE study’s primary endpoint.
Based on these results, Ionis plans to file a new drug application for the drug (NDA) with the FDA early next year. Investors were likely impressed with the results. Shares gained on the same as the FDA would expedite the filing’s review as olezarsen was granted fast-track designation in FCS indication earlier this year in January, assuming the NDA is filed.
Ionis also entered an agreement with Roche for two undisclosed early-stage programs for RNA-targeting investigational medicines for the treatment of Alzheimer's disease and Huntington's disease. Ionis will receive a $60 million upfront payment while Roche gains exclusive worldwide rights to develop, manufacture and commercialize the investigational medicines discovered by Ionis.
Soleno Announces Study Results: Soleno Therapeutics, Inc. SLNO announced positive top-line results on lead candidate DCCR (Diazoxide Choline) Extended-Release tablets for the treatment of Prader-Willi syndrome (PWS). The multi-center, randomized, double-blind, placebo-controlled randomized withdrawal period enrolled 77 patients previously enrolled in Study C602 who had been on open-label treatment with DCCR for between two and four years. Results from the study showed hyperphagia-related behaviors markedly worsened in the placebo group compared to DCCR, represented by a highly statistically significant, clinically meaningful difference in mean change from baseline in the HQ-CT total score of 5.0 at week 16.
Secondary endpoints of Clinical Global Impression of Severity and Clinical Global Impression of Improvement ratings both showed strong trends toward worsening in the placebo group compared to DCCR over the course of the randomized withdrawal period, respectively.
Following the positive results, Soleno plans to submit a NDA to the FDA by mid-2024 for DCCR to treat PWS.
The highly statistically significant data from the randomized withdrawal phase of Study C602 caused the company’s shares to soar on Sep 26 but the gains were pared the next day on profit booking by investors.
Performance
The Nasdaq Biotechnology Index has lost 0.59% in the past five trading sessions. Among the biotech giants, Moderna has lost 3.95% during the period. Over the past six months, shares of Moderna have plunged 32.97%. (See the last biotech stock roundup here: Biotech Stock Roundup: GSKs Drug Approval, MRNAs Vaccine News, PTCT & SPRY Down).
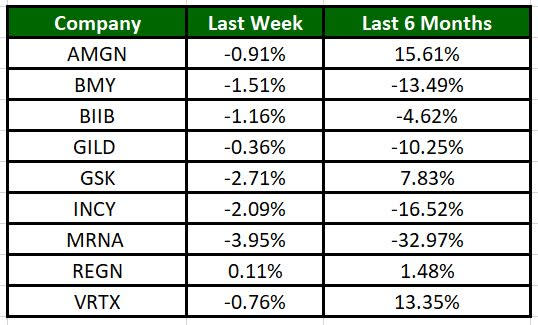
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for more pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Intercept Pharmaceuticals, Inc. (ICPT) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
Soleno Therapeutics, Inc. (SLNO) : Free Stock Analysis Report
Immunovant, Inc. (IMVT) : Free Stock Analysis Report
Pliant Therapeutics, Inc. (PLRX) : Free Stock Analysis Report
