bluebird (BLUE) Updates on Progress With Zynteglo, 2023 Outlook
bluebird bio, Inc. BLUE provides an update on the commercial launch progress of its gene therapies and its financial outlook for 2023.
bluebird received approvals for two of its gene therapies, Zynteglo (beti-cel) for transfusion-dependent thalassemia (TDT) and Syskona (eli-cel) for active cerebral adrenoleukodystrophy (CALD) in 2022.
Zynteglo was granted FDA approval in August as a one-time gene therapy customized for treating adults and pediatric patients with beta-thalassemia requiring regular red blood cell (RBC) transfusions across all genotypes. bluebird entered 2023 with multiple patient cell collections scheduled and first revenue anticipated for the first quarter as previously guided.
The company stated that approximately 40 patients have initiated benefits verification, with a significant proportion progressing to prior authorization approval across both Medicaid and commercial payer segments and zero ultimate denials to date.
Cell collection for the first patient to be treated commercially with Skysona is scheduled for January 2023.
Shares of bluebird have declined 19.9% in the past year compared with the industry’s decline of 18%.
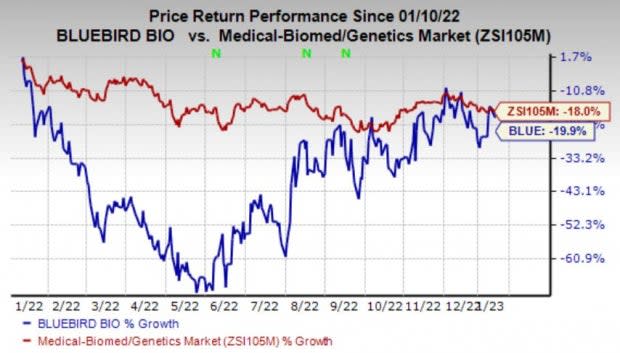
Image Source: Zacks Investment Research
bluebird stated completed vector and drug product analytical comparability studies in the fourth quarter of 2022 on lovotibeglogene autotemcel (lovo-cel), as previously announced. Lovotibeglogene autotemcel gene therapy is an investigational, one-time treatment being studied for sickle cell disease (SCD).
The company remains on track to submit its biologics license application (BLA) to the FDA this quarter. A potential approval of lovo cell will boost the growth prospects of the company.
As of Dec 31, 2022, cash and cash equivalents and marketable securities balance was approximately $182 million, excluding restricted cash of approximately $45 million. Cash burn is expected to be in the range of $270-$300 million.
bluebird bio, Inc. Price and EPS Surprise
bluebird bio, Inc. price-eps-surprise | bluebird bio, Inc. Quote
bluebird expects its cash, cash equivalents, restricted cash and marketable securities, including the proceeds from the sale of its second priority review voucher (PRV) for $95 million, will be sufficient to meet bluebird’s planned operating expenses and capital expenditure requirements into the first quarter of 2024.
While the therapies promise potential, gene therapies are complex by nature. Thus, Zynteglo and Syskona are pretty complex to administer and can only be provided as treatments to patients by qualified treatment centers, apart from being expensive treatments.
Hence, it remains to be seen how these therapies fare in the market.
Zacks Rank and Key Picks
bluebird currently has a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the biotech sector are Syndax Pharmaceuticals, Inc. SNDX and Inovio Pharmaceuticals INO, both carrying a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Syndax Pharmaceuticals for 2022 and 2023 have narrowed by 11 cents and 20 cents, respectively, over the past 60 days. Earnings of Syndax Pharmaceuticals surpassed estimates in three of the trailing four quarters and met the same on the other occasion. SNDX witnessed an earnings surprise of 95.39% on average.
Loss per share estimates for INO have narrowed by 29 cents and 36 cents for 2022 and 2023, respectively, in the past 60 days.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Inovio Pharmaceuticals, Inc. (INO) : Free Stock Analysis Report
bluebird bio, Inc. (BLUE) : Free Stock Analysis Report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report
