Boston Scientific (BSX) Initiates AVANT GUARD Clinical Trial
Boston Scientific, Inc. (BSX) initiated the AVANT GUARD clinical trial to evaluate the FARAPULSE Pulsed Field Ablation (“PFA”) System — a nonthermal treatment in which electric fields selectively ablate heart tissue in patients with atrial fibrillation (AF). The randomized clinical trial will study the safety and effectiveness of the FARAPULSE PFA System as a first-line treatment for persistent AF.
This is the only trial to study the use of PFA as frontline therapy in patients with this form of AF. The company also anticipates the FDA’s approval of the FARAPULSE PFA System in the first quarter of 2024.
Significance of the News
Persistent AF is sustained arrhythmia that lasts for more than a week, unlike paroxysmal AF, which describes symptoms that last for seven days or fewer. The early treatment of persistent AF can reduce the risk of blood clots, stroke and heart failure and may prevent the disease from becoming permanent. Patients are often treated with AADs as frontline therapy for heart rhythm maintenance, though some can experience adverse effects and limited efficacy.
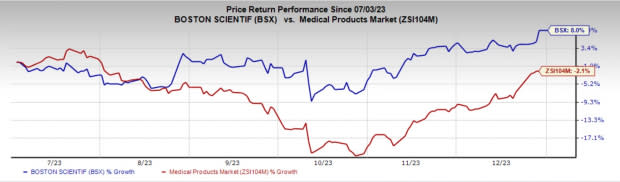
Image Source: Zacks Investment Research
Cardiac ablation is a potential alternative interventional strategy for those living with persistent AF. According to BSX’s representative of AF Solutions, the FARAPULSE PFA System has demonstrated a promising safety and effectiveness profile with nearly 40,000 patients treated to date in clinical and commercial settings.
News in Detail
Boston Scientific is excited about the potential of the AVANT GUARD trial to transform clinical practice. Advancing the therapy as an early treatment for persistent AF may lead to enhanced long-term outcomes, establishing the FARAPULSE PFA System as the preferred method for treating the disease.
The trial will enroll more than 500 patients diagnosed with persistent AF at up to 75 sites globally. Patients in the study will be randomized to undergo pulmonary vein isolation (PVI) and left atrial posterior wall ablation using the FARAPULSE PFA System or receive AAD treatment (an anti-arrhythmic drug commonly prescribed for patients living with persistent AF) and followed for three years. The trial will evaluate the outcomes of therapy provided with the FARAPULSE PFA System versus AADs, including device-or procedure-related adverse events, the rates of freedom from AF, atrial flutter or atrial tachycardia and AF burden — a measurement of the amount of AF an individual experiences.
All patients in the trial will also be inserted with the Boston Scientific LUX-Dx Insertable Cardiac Monitor. This device simplifies the monitoring process for patients by automatically capturing and transmitting arrhythmia episode data and is designed to detect the recurrence of cardiac arrhythmias and assess AF burden by providing continuous rhythm monitoring. Last week, the Cleveland Clinic enrolled the first patient in the AVANT GUARD trial.
Industry Prospects
Per a research report, the global AF Devices market was valued at $5.02 billion in 2022 and is expected to witness a CAGR of 13.8% by 2033.
Other Developments
At the ESC Congress 2023 (the annual meeting of the European Society of Cardiology), Boston Scientific presented positive 12-month results from the pivotal ADVENT clinical trial. The findings demonstrated that the FARAPULSE PFA System is non-inferior to standard-of-care therapies for the treatment of paroxysmal AF with superior efficiency. Additional real-world data from more than 17,000 patients simultaneously showed continued real-world safety, efficacy and efficiency of the system.
The company also completed enrollment in the first phase of the ADVANTAGE AF clinical trial in the third quarter of 2023, which is studying the system for the treatment of patients with drug-refractory symptomatic persistent AF.
Price Performance
In the past six months, Boston Scientific shares have risen 8% against the industry’s fall of 2.1%.
Zacks Rank and Key Picks
Boston Scientific currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Haemonetics HAE, Insulet PODD and DexCom DXCM. Haemonetics and DexCom each presently carry a Zacks Rank #2 (Buy), and Insulet sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Haemonetics’ stock has increased 8.7% in the past year. Earnings estimates for Haemonetics have remained constant at $3.89 in 2023 and at $4.15 in 2024 in the past 30 days.
HAE’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 16.1%. In the last reported quarter, it posted an earnings surprise of 5.3%.
Estimates for Insulet’s 2023 earnings per share have remained constant at $1.91 in the past 30 days. Shares of the company have dropped 26.3% in the past year against the industry’s rise of 3.7%.
PODD’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 105.1%. In the last reported quarter, it delivered an average earnings surprise of 77.4%.
Estimates for DexCom’s 2023 earnings per share have increased from $1.43 to $1.44 in the past 30 days. Shares of the company have increased 9.6% in the past year compared with the industry’s 3.8% rise.
DXCM’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 36.4%. In the last reported quarter, it delivered an average earnings surprise of 47.1%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Boston Scientific Corporation (BSX) : Free Stock Analysis Report
Haemonetics Corporation (HAE) : Free Stock Analysis Report
DexCom, Inc. (DXCM) : Free Stock Analysis Report
Insulet Corporation (PODD) : Free Stock Analysis Report
