BridgeBio (BBIO) Up as Heart Drug Meets Primary Goal in Study
BridgeBio BBIO soared almost 76% on Monday, as its late-stage study of acoramidis in patients with transthyretin amyloid cardiomyopathy (ATTR-CM) met its primary endpoint with high statistical significance.
The phase III study called ATTRibute-CM evaluated acoramidis as a next-generation, orally administered small molecule that can act as a highly potent stabilizer of transthyretin.
The primary endpoint of the study was determined through a hierarchical analysis. After 30 months, the study showed 81% survival rate among patients who received acoramidis, compared with 74% among those in the placebo group. In addition, there was a significant relative risk reduction of 50% in the frequency of cardiovascular-related hospitalizations.
The study also showed a consistent positive treatment effect at 30 months across additional markers of morbidity, quality of life and function.
No sign of concern was observed during the study, indicating a favorable safety profile for acoramidis.
BridgeBio’s shares have rallied almost 320.5% in the year-to-date period against the industry's 14.8% decline.
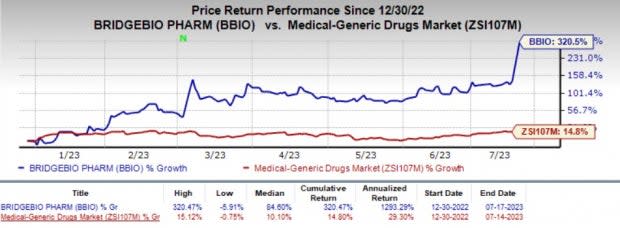
Image Source: Zacks Investment Research
The company now plans to submit the new drug application for acoramidis to the FDA by the end of 2023. BBIO also intends to submit, regulatory filings in additional markets by 2024.
ATTR-CM is one of the leading causes of heart failure, characterized by the accumulation of abnormal proteins on the heart. If successfully approved, acoramidis will be competing with Pfizer’s approved drugs — Vyndamax and Vyndaqel — for treating ATTR-CM.
Alnylam Pharmaceuticals ALNY is currently in the process of seeking regulatory approval in the United States to broaden the application of its drug Onpattro to include the treatment of cardiomyopathy. A decision on this expansion is anticipated to be made by October 2023. Onpattro is currently approved for a specific type of amyloidosis that primarily impacts the nerves.
BridgeBio’s commercial products include Nulibry, approved to treat patients with molybdenum cofactor deficiency (MocD) type A. It is the first and only FDA-approved therapy that can reduce the risk of death in patients with MocD Type A.
BridgeBio Pharma, Inc. Price and Consensus
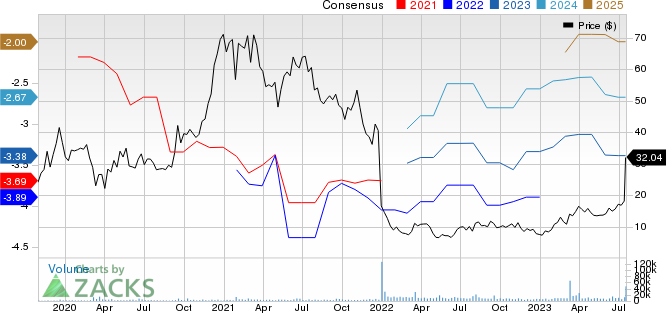
BridgeBio Pharma, Inc. price-consensus-chart | BridgeBio Pharma, Inc. Quote
Zacks Rank & Stocks to Consider
BridgeBio currently carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the overall healthcare sector are Omega Therapeutics OMGA and Alkermes ALKS, both sporting a Zacks Rank #1 (Strong Buy) at present.You can see the complete list of today’s Zacks #1 Rank stocks here.
The Zacks Consensus Estimate for Omega Therapeutics has narrowed from a loss of $2.49 per share to a loss of $2.05 for 2023 in the past 90 days. Shares of the company have lost 7.9% year to date.
OMGA’s earnings beat estimates in two of the trailing four quarters, met the mark in one and missed in another, delivering an average surprise of 8.24%.
The bottom-line estimate for Alkermes has gone up from 7 cents to $1.02 for 2023 in the past 90 days. The company's shares have rallied 17% year to date.
ALKS’ earnings beat estimates in three of the trailing four quarters and met the mark in one, delivering an average surprise of 90.83%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
Alkermes plc (ALKS) : Free Stock Analysis Report
BridgeBio Pharma, Inc. (BBIO) : Free Stock Analysis Report
Omega Therapeutics, Inc. (OMGA) : Free Stock Analysis Report
