Bristol Myers (BMY) Gets EC Nod for Abecma's Label Expansion
Bristol Myers BMY announced that the European Commission (EC) has approved a label expansion for chimeric antigen receptor (CAR) T cell immunotherapy Abecma.
The therapy is now approved in the European Union (EU) in earlier lines for triple-class exposed relapsed and refractory multiple myeloma.
Abecma is already approved in the EU, Switzerland, Japan, the United Kingdom and Israel for adult patients with triple-class exposed relapsed or refractory multiple myeloma after three to four or more prior lines of therapy.
The latest approval makes Abecma the first CAR T cell immunotherapy approved in the EU for use in earlier lines of therapy for relapsed and refractory multiple myeloma.
The EC approval is based on results from the phase III KarMMa-3 study, wherein Abecma demonstrated superiority over standard regimens with a 51% reduction in risk of disease progression or death and a well-established safety profile with mostly low-grade and transient occurrences of cytokine release syndrome and neurotoxicity.
An approval in the EU was mostly expected as the Committee for Medicinal Products for Human Use of the European Medicines Agency had earlier given a positive opinion, recommending Abecma’s approval for this patient class.
It is to be noted that Abecma is being jointly developed and commercialized in the United States between Bristol Myers Squibb and 2seventy bio TSVT. Outside of the United States, BMY assumes the sole responsibility for Abecma’s manufacturing and commercialization.
Last week, Bristol Myers and partner 2seventy bio announced that the FDA Oncologic Drugs Advisory Committee (ODAC) voted in favor (8:3) of Abecma’s benefit/risk profile for patients with triple-class exposed relapsed or refractory multiple myeloma.
The favorable voting was also based on results from the KarMMa-3 study, including the key secondary endpoint of overall survival (OS).
Abecma’s sales totaled $472 million in 2023, up 22% year over year. Label expansion of the therapy will further boost its sales.
Shares of Bristol Myers have plunged 22.8% in the past year compared with the industry’s decline of 6.6%.
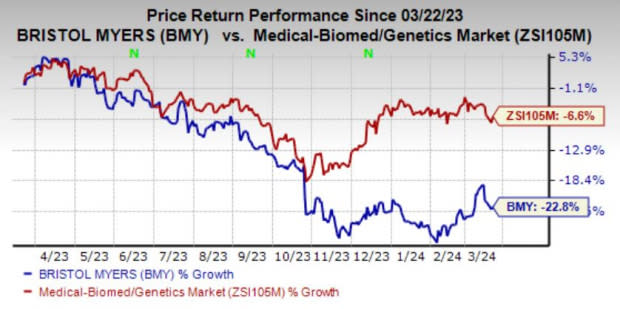
Image Source: Zacks Investment Research
BMY recently obtained FDA’s accelerated approval for its other CAR T cell therapy, Breyanzi (lisocabtagene maraleucel), for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma, who have received at least two prior lines of therapy, including a Bruton tyrosine kinase inhibitor and a B-cell lymphoma 2 inhibitor.
Breyanzi is already approved in the United States, Japan and Europe for the second-line treatment of relapsed or refractory large B-cell lymphoma.
However, CAR T cell therapies have been under FDA scrutiny for the past few months. The FDA earlier asked Gilead Sciences, Inc., Johnson & Johnson and Novartis to add “boxed warning” to the labels of their T-cell immunotherapies, per Reuters.
The agency has determined that the risk of T-cell malignancies is applicable to all currently approved BCMA-directed and CD19-directed genetically modified autologous CAR T-cell immunotherapies, including BMY’s Abecma and Breyanzi.
Concurrently, Bristol Myers announced that the phase III CheckMate -9DW trial evaluating dual immunotherapy combination of Opdivo (nivolumab) plus Yervoy (ipilimumab) as a first-line treatment for patients with advanced hepatocellular carcinoma (HCC) who have not received prior systemic therapy met its primary endpoint of improved OS compared to investigator’s choice of Nexavar (sorafenib) or lenvatinib at a pre-specified interim analysis.
Please note that Opdivo, in combination with Yervoy, is already indicated for the treatment of adult patients with HCC who have been previously treated with Nexavar. This indication is approved under accelerated approval based on the overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Bristol Myers Squibb Company Price, Consensus and EPS Surprise
Bristol Myers Squibb Company price-consensus-eps-surprise-chart | Bristol Myers Squibb Company Quote
Currently, Opdivo is approved both as a monotherapy and in combination with Yervoy to treat a plethora of cancer indications in many countries, including the United States and the EU.
Approval of new drugs and label expansion of existing ones is important for BMY as it looks to offset the decline in Revlimid and Eliquis’ revenues.
Opdivo maintains momentum for the company and new drugs like Opdulag have witnessed robust uptake too. However, the loss of Revlimid revenues weighs on the top line.
Zacks Rank & a Stock to Consider
Bristol Myers currently carries a Zacks Rank #4 (Sell).
A couple of better-ranked stocks in the healthcare sector are ADMA Biologics, Inc. ADMA and ANI Pharmaceuticals, Inc. ANIP, both sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share have improved from 22 to 30 cents. In the past year, shares of ADMA have rallied 109.9%.
ADMA Biologics’ earnings beat estimates in three of the trailing four quarters and met the same once, delivering an average surprise of 85.00%.
In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 earnings per share have improved from $4.06 to $4.40. In the past year, shares of ANIP have surged 75.8%.
ANI Pharmaceuticals’ earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 109.06%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
2seventy bio, Inc. (TSVT) : Free Stock Analysis Report
