Bristol Myers' (BMY) sBLAs for Breyanzi Get FDA Priority Tag
Bristol Myers Squibb Company BMY announced that the FDA has accepted its two supplemental biologics license applications (sBLA) seeking label expansion for Breyanzi in two new indications. The sBLAs are seeking approval for Breyanzi for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) and relapsed or refractory mantle cell lymphoma (MCL) after a Bruton tyrosine kinase inhibitor (BTKi).
Breyanzi is a CD19-directed CAR T cell therapy with a 4-1BB costimulatory domain, which enhances the expansion and persistence of the CAR T cells.
With the FDA granting a priority review to both sBLAs for Breyanzi, a decision from the regulatory body is expected on May 23, 2024 for the relapsed/refractory FL indication and May 31, 2024 for the relapsed/refractory MCL indication.
Simultaneously, the company announced that Japan’s Ministry of Health, Labour and Welfare (MHLW) has also accepted the supplemental new drug application (sNDA) for Breyanzi for the treatment of relapsed or refractory FL.
For the FL indication, the sBLA for Breyanzi in the United States and the sNDA in Japan were based on data from the phase II TRANSCEND FL study, which evaluated the safety and efficacy of Breyanzi in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma (NHL), including FL and marginal zone lymphoma. Data from the study showed that treatment with Breyanzi demonstrated high rates of complete responses.
For the MCL indication, the sBLA for Breyanzi in the United States was based on data from the phase I TRANSCEND NHL 001 study, which evaluated Breyanzi in patients with relapsed or refractory B-cell NHL, including diffuse large B-cell lymphoma, high-grade B-cell lymphoma, primary mediastinal B-cell lymphoma, FL Grade 3B and mantle cell lymphoma. Data from this study showed that treatment with Breyanzi led to statistically significant and clinically meaningful responses in heavily pre-treated patients, with the majority of patients achieving a complete response.
Shares of Bristol Myers have plunged 30.6% in the past year compared with the industry’s decline of 12%.
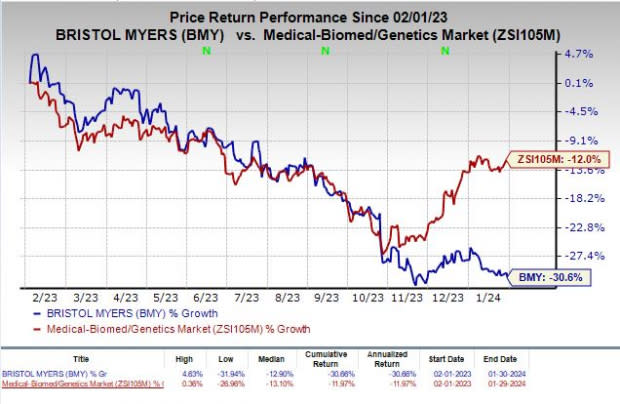
Image Source: Zacks Investment Research
Meanwhile, another sBLA seeking approval for Breyanzi for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma who have received a prior BTKi and B-cell lymphoma 2 inhibitor is also under priority review in the United States. A decision from the FDA is due on Mar 14, 2024.
Breyanzi is approved by the FDA for the treatment of adult patients with large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma, primary mediastinal LBCL and follicular lymphoma grade 3B, who have refractory disease to first-line chemoimmunotherapy or relapse within 12 months of first-line chemoimmunotherapy, or refractory disease to first-line chemoimmunotherapy or relapse after first-line chemoimmunotherapy and are not eligible for hematopoietic stem cell transplant due to comorbidities or age or relapsed or refractory disease after two or more lines of systemic therapy.
Breyanzi generated sales worth $263 million in the first nine months of 2023, reflecting a significant increase year over year.
The approval of new drugs and label expansion of existing ones will enable Bristol Myers to diversify its product base and drive top-line growth in the days ahead.
Zacks Rank & Stocks to Consider
Bristol Myers currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the healthcare sector are ADMA Biologics, Inc. ADMA, Akoya Biosciences, Inc. AKYA and Puma Biotechnology, Inc. PBYI, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share have improved from 16 cents to 22 cents. In the past year, shares of ADMA have rallied 41.9%.
Earnings of ADMA Biologics beat estimates in three of the last four quarters and met the same once. ADMA delivered a four-quarter average earnings surprise of 63.57%.
In the past 60 days, estimates for Akoya Biosciences’ 2024 loss per share have narrowed from $1.01 to 92 cents. In the past year, shares of AKYA have plunged 57.5%.
Akoya Biosciences beat estimates in one of the last four quarters while missing the same on the remaining three occasions. AKYA delivered a four-quarter negative earnings surprise of 3.66%, on average.
In the past 60 days, estimates for Puma Biotechnology’s 2024 earnings per share have improved from 62 cents to 69 cents. In the past year, shares of PBYI have risen 9.5%.
Earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Akoya Biosciences, Inc. (AKYA) : Free Stock Analysis Report
