CAR T-Cell Therapies Under FDA Watch on Reports of Malignancies
The FDA recently announced that it is investigating the identified risk of T-cell malignancy in patients who received treatment with chimeric antigen receptor (CAR) T-cell immunotherapies. These malignancies have been reported in patients treated with almost all products in this class.
It is a matter of grave concern if a particular class of treatment for cancer results in lymphoma. The FDA has, therefore, rightly initiated the investigation. The agency also stated that it would take regulatory action if needed.
The investigation comes on the heels of reports received by the FDA of T-cell malignancies in patients who received treatment with BCMA- or CD19-directed autologous CAR T-cell immunotherapies. The malignancy resulted in not just hospitalization of patients but, in certain cases, death. These reports came from ongoing clinical trials and/or postmarketing adverse event (AE) data sources.
The agency has determined that the risk of T-cell malignancies is applicable to all currently approved BCMA-directed and CD19-directed genetically modified autologous CAR T-cell immunotherapies. The FDA has approved six CAR T cell immunotherapies so far. These include Bristol Myers’ BMY Abecma (idecabtagene vicleucel) and Breyanzi (lisocabtagene maraleucel), Gilead Sciences’ GILD Yescarta (axicabtagene ciloleucel) and Tecartus (brexucabtagene autoleucel), Novartis’ NVS Kymriah, and Janssen and Legend Biotech’s LEGN Carvykti (ciltacabtagene autoleucel).
Most of these therapies are approved for various forms of blood cancers.
CAR-T cell therapy is a type of cellular immunotherapy, which involves using a patient’s own immune cells to attack and get rid of harmful disease cells in the body. CAR T uses the patient’s cells to identify and destroy cancer cells, thereby making it different from other small molecule or biological therapies. During the treatment, T cells are drawn from a patient's blood. These cells are then reprogrammed in the manufacturing facility to create genetically coded cells that are injected back into the patient. These then recognize and fight cancer cells.
There is a lot of enthusiasm for CAR T-cell therapies, given the significant need for many cases where surgery, chemotherapy and radiation therapy fail to keep the disease under control. Early-stage studies showed that CAR T-cell therapies help achieve durable, complete responses in some leukemias and lymphomas, including in patients who have suffered multiple relapses.
While the benefits of these CAR T-cell therapies continue to outweigh the potential risks for their approved uses, it seems the long-term impact of the treatment still needs to be evaluated.
Patients and clinical trial participants receiving treatment with these products need to be monitored life-long for new malignancies.
Such risks are, however, not uncommon and unheard of. We remind investors that all gene therapy products with integrating vectors (lentiviral or retroviral vectors) carry the potential risk of developing secondary malignancies. This risk is also labeled as a class warning in the U.S. prescribing information for approved BCMA-directed and CD19-directed genetically modified autologous T-cell immunotherapies.
Per AE reports, there have been T-cell lymphoma cases for Novartis’ Kymriah, Gilead’s Yescarta, Bristol Myers’ Breyanzi and Legend Biotech's Carvykti.
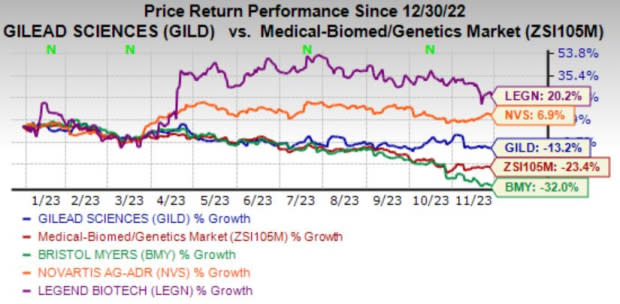
Image Source: Zacks Investment Research
Among these, Novartis’ Kymriah was the first to get approval in 2017 for the treatment of patients up to 25 years of age with B cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Gilead acquired Kite Pharma to foray into the emerging field of cell therapy. Kite’s Yescarta received approval for treating refractory aggressive non-Hodgkin lymphoma, including diffuse large B-cell lymphoma, transformed follicular lymphoma and primary mediastinal B-cell lymphoma, which was a significant boost for the company. Approval for additional indications will further boost growth. The company has one more therapy in its quiver, Tecartus, which is approved for the treatment of adult patients with relapsed or refractory mantle cell lymphoma. Both these therapies have helped Gilead to create a presence in this space.
Bristol Myers is developing Abecma with partner 2seventy bio, Inc. Abecma is a CAR T-cell therapy indicated for adult patients with triple-class exposed relapsed or refractory multiple myeloma after four or more prior lines of therapy. Both these companies are also looking to get Abecma approved for earlier lines of therapy as well.
Legend Biotech earns collaboration revenues on product sales of Carvykti from Janssen. This CAR-T cell therapy was approved last year for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy.
The investigation news caused some worry among investors as LEGN lost 2.6%. Novartis, Bristol Myers and Gilead too witnessed a slight decline in share price.
The stocks currently carry a Zacks Rank #3 (Hold) each. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Legend Biotech Corporation Sponsored ADR (LEGN) : Free Stock Analysis Report
