Centessa (CNTA) Surges 72% in the Past 3 Months: Here's Why
Centessa Pharmaceuticals CNTA, a clinical-stage company, is currently developing and advancing two developmental candidates, one in a registrational study and another in an early-mid-stage study, to change the current treatment paradigm and establish a new standard of care.
The company’s lead product candidate, SerpinPC, is being evaluated in a registrational PRESent-2 study for the treatment of hemophilia B without inhibitors. SerpinPC is an investigational and subcutaneously-administered novel activated protein C inhibitor. Centessa’s second clinical-stage candidate, LB101, a PD-L1xCD47 LockBody, is being developed in a phase I/IIa study for solid tumor indications.
Shares of Centessa have skyrocketed about 72.2% in the last three months compared with the industry’s 0.1% rise. The significant surge in the stock price is primarily attributable to the company’s solid progress in the registrational study of its lead candidate, along with corporate updates provided regarding its pipeline, in Centessa’s second-quarter 2023 earnings release.
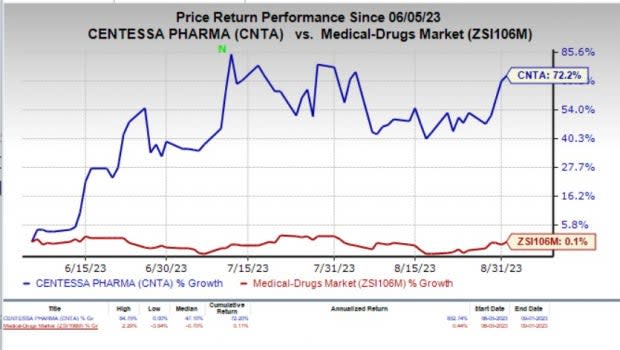
Image Source: Zacks Investment Research
In July 2023, CNTA announced dosing the first patient in its registrational PRESent-2 study of SerpinPC for the treatment of hemophilia B without inhibitors. Following the dosing phase of the PRESent-2 study, prospective baseline data regarding the patient’s disease status will be collected during a 12-week observation period to assess the benefit/risk profile of the drug for regulatory approval.
Management believes that SerpinPC has the potential to become a new standard of care in the treatment of hemophilia B and a differentiated safety profile, which is based on the encouraging results from its ongoing phase IIa study of SerpinPC.
Per Centessa, the registrational study intends to enroll and dose additional patients, conducting a planned interim analysis, when 36 subjects reach the stipulated 12 weeks of treatment with SerpinPC in Part 1 of the study.
Centessa is also planning to initiate dosing in its registrational PRESent-3 study for patients with hemophilia B with inhibitors by the end of 2023.
SerpinPC currently enjoys the FDA’s Fast Track Designation in the United States for the treatment of hemophilia B, with or without inhibitors. To date, the candidate has not been approved by the FDA or any other regulatory body.
In its second-quarter earnings release, CNTA also reported continuing enrollment and dosing patients in its phase I/IIa study of LB101 for the treatment of solid tumors. The company further announced its second LockBody candidate, LB206, which is currently in pre-clinical studies.
In the second quarter of 2023, Centessa reported a loss of 26 cents per share, narrower than the Zacks Consensus Estimate of a loss of 43 cents. The better-than-expected financial performance of the company during the reported quarter is likely to have also contributed to the soaring of the stock price.
Furthermore, the company also enjoys a comfortable cash position of $303.6 million in cash, cash equivalents and short-term investments, as of June-end 2023, which is expected to fund the company’s operations into 2026.
In the absence of a marketed product, Centessa did not generate any revenues during the second quarter.
Centessa Pharmaceuticals PLC Sponsored ADR Price and Consensus
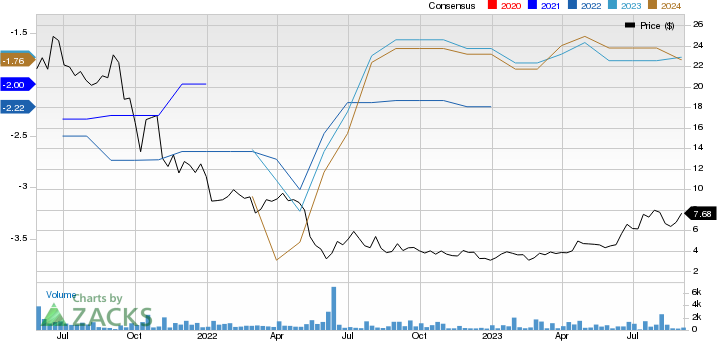
Centessa Pharmaceuticals PLC Sponsored ADR price-consensus-chart | Centessa Pharmaceuticals PLC Sponsored ADR Quote
Zacks Rank and Stocks to Consider
Centessa currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the pharma/biotech sector are Dynavax Technologies DVAX, Better Therapeutics BTTX and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Dynavax’s 2023 loss per share has narrowed from 51 cents to 24 cents. The estimate for Dynavax’s 2024 earnings per share is currently pegged at 2 cents. In the past three months, shares of DVAX have risen by 25.9%.
DVAX’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 25.78%.
In the past 30 days, the Zacks Consensus Estimate for Better Therapeutics’ 2023 loss per share has narrowed from $1.33 to $1.28. During the same period, Better Therapeutics’ 2024 loss per share has also narrowed from 83 cents to 80 cents. In the past three months, shares of BTTX have lost 16.1%.
BTTX’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 24.22%.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has gone up from 69 cents to 78 cents. During the same period, the estimate for Corcept’s 2024 earnings per share has also improved from 69 cents to 83 cents. In the past three months, shares of CORT have climbed 42.5%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
Centessa Pharmaceuticals PLC Sponsored ADR (CNTA) : Free Stock Analysis Report
Better Therapeutics, Inc. (BTTX) : Free Stock Analysis Report
