Charles River's (CRL) Memphis Site Achieves Crucial Approval
Charles River Laboratories International, Inc. CRL recently achieved an important milestone in its strategic collaboration with Vertex Pharmaceuticals to manufacture CASGEVY (exagamglogene autotemcel [exa-cel]). The company’s Memphis facility has been approved to manufacture Vertex’s CASGEVY — the first-ever gene-edited therapy in the world that targets severe sickle cell disease (SCD).
As a cell therapy CDMO (contract development and manufacturing organization), the recent development will boost Charles River’s Cell Therapy Manufacturing Services. The company offers cell and gene-modified cell therapy developers a robust and scalable process to swiftly transition autologous and allogeneic programs to clinic and commercialization with one production partner.
Significance of the CASGEVY
Vertex Pharmaceuticals collaborated with CRISPR Therapeutics to leverage the use of a gene-editing technology known as CRISPR/Cas9 to discover and develop CASGEVY. Earlier this month, it became the first CRISPR-based gene-editing therapy to be approved in the United States for the treatment of SCD. Approximately 16,000 patients aged 12 and above with severe SCD may now qualify for this one-time treatment that potentially offers a functional cure for their disease by eliminating severe VOCs (vaso-occlusive crises) and hospitalizations caused by severe VOCs.
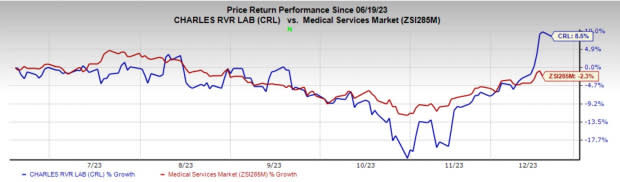
Image Source: Zacks Investment Research
SCD is an inherited blood disease impacting millions of people worldwide. SCD affects hemoglobin, a part of the blood that carries oxygen around the body. People who suffer from this condition require lifelong treatment and significant use of healthcare resources, ultimately resulting in reduced life expectancy.
More on the News
Charles River’s Memphis team expressed delight in securing the regulatory approval to manufacture CASGEVY. The Memphis facility was the first CDMO in North America to be approved by the EMA (European Medicines Agency) to commercially manufacture an allogeneic cell therapy drug product. The Memphis center of excellence passed back-to-back audits from the FDA and the Health Products Regulatory Authority on behalf of the EMA.
Together with Vertex Pharmaceuticals, the company is pleased to achieve this milestone of manufacturing the world’s first gene-edited therapy. Given the tremendous need for this treatment, the collaboration looks forward to helping bring CASGAVY to patients.
Industry Prospects
Per a Research report, the global gene-editing market was valued at $6.9 billion in 2022 and is expected to witness a CAGR of 15.7% by 2032.
Progress in Cell and Gene Therapy
In recent years, Charles River has significantly broadened its cell and gene therapy portfolio. This includes a substantial good manufacturing practice (GMP)-compliant commercial-ready capacity expansion and the integration of several strategic acquisitions to simplify complex supply chains and meet the growing demand for plasmid DNA, viral vector and cell therapy.
Last month, the company announced a gene therapy manufacturing collaboration with the Australian non-profit foundation, Genetic Cures for Kids Inc. Under the partnership, the company will perform plasmid DNA production in support of early-phase trials for Hereditary Spastic Paraplegia Type 56 (SPG56) — a progressive neurological disease characterized by varying degrees of spasticity and muscle weakness without any available cure.
Charles River also introduced an expanded CliniPrime suite of GMP-compliant offerings with the launch of CliniPrime Cryopreserved Leukopaks for cell therapy development and manufacturing. The new CliniPrime Cryopreserved Leukopak offering provides a solution to a growing industry’s need for reliable and consistent sources of cellular starting material.
Price Performance
Over the past six months, Charles River shares have risen 8.5% against the industry’s decline of 2.3%.
Zacks Rank and Key Picks
Charles River currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Haemonetics HAE, Insulet PODD and DexCom DXCM. Haemonetics and DexCom each presently carry a Zacks Rank #2 (Buy), and Insulet sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Haemonetics’ stock has risen 12.6% in the past year. Earnings estimates for Haemonetics have increased from $3.86 to $3.89 in 2023 and $4.11 to $4.15 in 2024 in the past 30 days.
HAE’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 16.1%. In the last reported quarter, it posted an earnings surprise of 5.3%.
Estimates for Insulet’s 2023 earnings per share have moved up from $1.90 to $1.91 in the past 30 days. Shares of the company have dropped 29.8% in the past year compared with the industry’s decline of 1.3%.
PODD’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 105.1%. In the last reported quarter, it delivered an average earnings surprise of 77.4%.
Estimates for DexCom’s 2023 earnings per share have increased from $1.43 to $1.44 in the past 30 days. Shares of the company have increased 9% in the past year compared with the industry’s rise of 3.9%.
DXCM’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 36.4%. In the last reported quarter, it delivered an average earnings surprise of 47.1%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Haemonetics Corporation (HAE) : Free Stock Analysis Report
DexCom, Inc. (DXCM) : Free Stock Analysis Report
Charles River Laboratories International, Inc. (CRL) : Free Stock Analysis Report
Insulet Corporation (PODD) : Free Stock Analysis Report
