CHMP to Delay Nod on Biogen (BIIB), Eisai's Alzheimer's Drug
Biogen BIIB and partner Eisai recently announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has delayed the decision on their Alzheimer’s disease (“AD”) drug lecanemab.
Per the companies, this delay is entirely related to procedural reasons at the EMA and is not related to the marketing authorization application (MAA) submitted for lecanemab. This MAA has been under EMA’s review since January 2023.
This delay is in consequence of an EU court ruling passed on Mar 14 that had "implications on EMA's policy on the handling of competing interests of experts." Due to this reason, the EMA also had to call off the advice it received at the Scientific Advisory Group on Neurology (SAG-N) meeting for lecanemab held on Mar 11. The EU regulator intends to reconvene another SAG-N meeting but has yet to schedule it.
Biogen has developed Leqembi in collaboration with Eisai, with the latter leading the clinical development and regulatory submissions. Though the companies co-commercialize and co-promote the drug, Eisai has the final decision-making authority.
The Biogen-Eisai partnered drug is currently approved for AD indication in the United States, Japan and China. It is being marketed under the trade name Leqembi.
In the year so far, Biogen’s stock has lost 16.3% compared with the industry’s 1.0% decline.
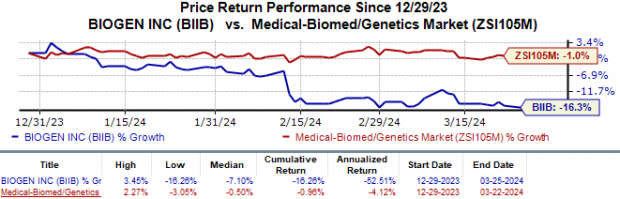
Image Source: Zacks Investment Research
The MAA is supported by data from the phase III CLARITY-AD study, which achieved its primary endpoint of reduction in the Clinical Dementia Rating-Sum of Boxes (“CDR-SB”) scale by 27% compared with a placebo. The CDR-SB is a numerical scale that measures the severity of dementia symptoms. The study also achieved statistically significant results for its secondary endpoints.
Last July, the FDA granted full approval to Leqembi in AD indication. Following approval, the Biogen/Eisai drug became the first and only approved anti-amyloid antibody treatment shown to reduce the rate of disease progression and slow cognitive impairment in the early and mild dementia stages of AD indication.
After receiving full/standard approval from the FDA, it also gained eligibility for broader Medicare coverage. Such coverage is crucial for a wider rollout of treatment. Though Leqembi sales were slow in 2023, Biogen/Eisai expects the same to start growing this year.
Leqembi is the first and only approved anti-amyloid antibody treatment in the United States, which has been shown to reduce the rate of disease progression and slow cognitive impairment in the early and mild dementia stages of AD indication.
The AD target market is highly competitive as several other pharma companies like Eli Lilly LLY and Prothena PRTA are developing their antibody candidates targeting the AD indication. The Alzheimer’s drugs of these companies are either under regulatory review or being evaluated in clinical studies.
Eli Lilly developed donanemab, its antibody therapy for AD, which is currently under review by the FDA. Earlier this month, Lilly announced that the FDA will delay its decision on the donanemab filing, which was initially expected before the end of first-quarter 2024. The agency intends to convene an advisory committee meeting to discuss data from the phase III TRAILBLAZER-ALZ 2 study, which supports Lilly’s regulatory filing for donanemab. A date for this meeting is yet to be determined.
Prothena is evaluating multiple AD candidates in early-stage studies targeting AD indication. Prothena is evaluating PRX005, an investigational antibody that targets tau, a protein implicated in diseases — including AD, frontotemporal dementia, progressive supranuclear palsy, chronic traumatic encephalopathy and other tauopathies. The candidate is being developed in collaboration with Bristol Myers.
Prothena is also evaluating another investigational high-potency monoclonal antibody, PRX012, designed to target a key epitope at the N-terminus of Aβ for treating AD in a phase I study. PRTA also plans to initiate an early-stage study for PRX123, a dual Aβ-Tau vaccine treatment and prevention therapy for AD. An update on both candidates is expected later this year.
Biogen Inc. Price
Biogen Inc. price | Biogen Inc. Quote
Zacks Rank & A Key Pick
Biogen currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is ANI Pharmaceuticals ANIP, which sports a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 EPS have risen from $4.06 to $4.43. Meanwhile, during the same period, EPS estimates for 2025 have improved from $4.80 to $5.04. Year to date, shares of ANIP have risen 26.2%.
Earnings of ANI Pharmaceuticals beat estimates in each of the last four quarters. ANI delivered a four-quarter average earnings surprise of 109.06%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Prothena Corporation plc (PRTA) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
