Clearside Biomedical Inc (CLSD) Reports Significant Licensing Revenue Growth in Q4 and Full ...
License Revenue: Q4 revenue soared to $6.3 million, driven by a $5.0 million upfront license fee from BioCryst.
R&D Investments: R&D expenses increased to $6.3 million in Q4 due to ODYSSEY clinical trial costs.
Net Loss Improvement: Q4 net loss narrowed to $4.8 million ($0.08 per share) from $9.7 million ($0.16 per share) in Q4 2022.
Cash Position: Cash and cash equivalents stood at $28.9 million at year-end, with funding expected to last into Q3 2025.
Clinical Progress: Positive clinical data reported from partner programs using the SCS Microinjector.
On March 12, 2024, Clearside Biomedical Inc (NASDAQ:CLSD) released its 8-K filing, detailing the financial results for the fourth quarter and the full year of 2023. The company, a pioneer in the field of biopharmaceuticals, is focused on developing therapies for blinding eye diseases through its proprietary SCS Microinjector. This innovative platform enables targeted delivery of treatments directly to the back of the eye, a method that has the potential to revolutionize the standard of care in ophthalmology.
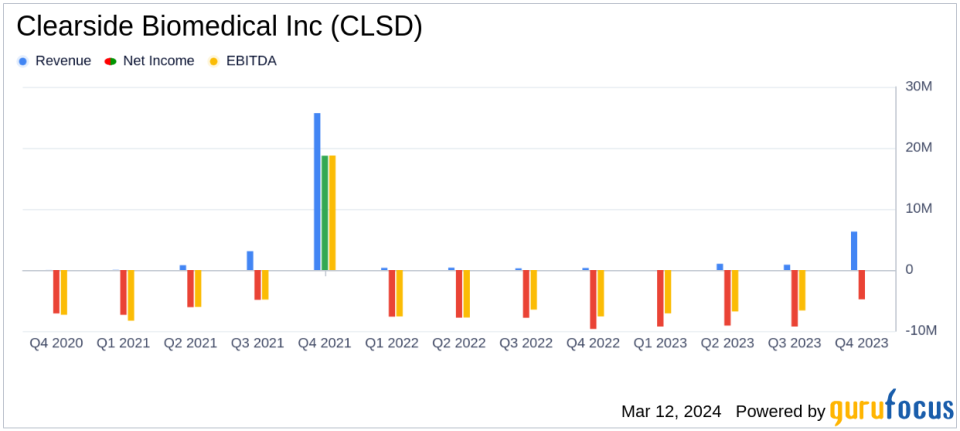
Financial Performance and Corporate Developments
Clearside Biomedical reported a substantial increase in license revenue for the fourth quarter, jumping to $6.3 million, up from $0.3 million in the same period of the previous year. This increase was largely due to a $5.0 million upfront license fee from BioCryst Pharmaceuticals and a $1.0 million milestone payment from Aura Biosciences. The company's R&D expenses also rose to $6.3 million, reflecting the costs associated with the ODYSSEY clinical trial for its lead product candidate, CLS-AX, in wet AMD.
Despite these increased expenses, the net loss for the quarter improved, decreasing to $4.8 million, or $0.08 per share, compared to a net loss of $9.7 million, or $0.16 per share, in the fourth quarter of 2022. This improvement was primarily due to the significant license revenue received during the quarter.
Strategic Partnerships and Clinical Advancements
Clearside's strategic partnerships have been a highlight, with the company entering into an exclusive worldwide license with BioCryst Pharmaceuticals for the delivery of their proprietary plasma kallikrein inhibitor, avoralstat, for the treatment of DME. This agreement, along with a successful equity offering, has bolstered the company's capital position, enabling it to fund operations into the third quarter of 2025.
The company's clinical progress has been marked by positive data from partner programs utilizing the SCS Microinjector. Notably, presentations at medical meetings have showcased the safety and efficacy of Clearside's suprachoroidal delivery platform, including data on CLS-AX in wet AMD and the extended treatment duration of XIPERE.
Looking Ahead
Clearside Biomedical's focus remains on the completion of the ODYSSEY Phase 2b clinical trial, with topline data expected in Q3 2024. The company's management expressed confidence in the potential of CLS-AX to become a best-in-class product for long-term maintenance therapy for wet AMD patients.
With a strengthened capital position and a pipeline of promising product candidates, Clearside Biomedical is well-positioned to continue its mission of improving vision for patients with sight-threatening eye diseases. Investors and stakeholders are encouraged to follow the company's progress as it advances its clinical programs and leverages its unique delivery platform to bring innovative treatments to market.
For more detailed financial information and corporate updates, interested parties can access the webcast and conference call hosted by Clearside's management.
Conclusion
Clearside Biomedical Inc (NASDAQ:CLSD) has demonstrated a strong financial quarter, underpinned by strategic licensing agreements and clinical advancements. The company's focus on its SCS Microinjector technology and the development of CLS-AX positions it at the forefront of innovation in ophthalmic treatments. With a solid cash position and a clear strategic direction, Clearside Biomedical is poised for continued success in the biopharmaceutical industry.
Explore the complete 8-K earnings release (here) from Clearside Biomedical Inc for further details.
This article first appeared on GuruFocus.
