Corcept (CORT) Begins Phase II Study on Dazucorilant for ALS
Corcept Therapeutics Incorporated CORT recently announced that it has initiated a phase II study – DAZALS – evaluating its pipeline candidate, dazucorilant, for the treatment of patients with amyotrophic lateral sclerosis (ALS), a destructive neuromuscular illness.
The double-blind, placebo-controlled phase II DAZALS study is investigating dazucorilant, a selective cortisol modulator, in patients with ALS. The primary endpoints of the study are to check the ALS Functional Rating Scale-Revised total score and safety, while key secondary endpoints include overall survival and quality of life.
Corcept is conducting the DAZALS study in collaboration with TRICALS, the leading ALS academic consortium in Europe. The same is being conducted to understand the potential of dazucorilant to significantly improve treatment outcomes for people living with ALS.
If successfully developed and approved, dazucorilant might offer a better treatment option for ALS, a disease that has an urgent need for better treatment.
Other companies are developing treatments for addressing ALS as well. Apellis Pharmaceuticals APLS is evaluating systemic pegcetacoplan in the phase II MERIDIAN study for treating ALS. Top-line data from the same is expected in mid-2023.
Apellis received FDA approval for pegcetacoplan as a monotherapy treatment for adult patients suffering from paroxysmal nocturnal hemoglobinuria in May 2021. APLS markets pegcetacoplan under the trade name Empaveli in the United States.
A potential approval for pegcetacoplan in ALS might induce acute competition for Corcept in the future.
Shares of Corcept have rallied 33.9% so far this year against the industry’s decline of 29.7%.
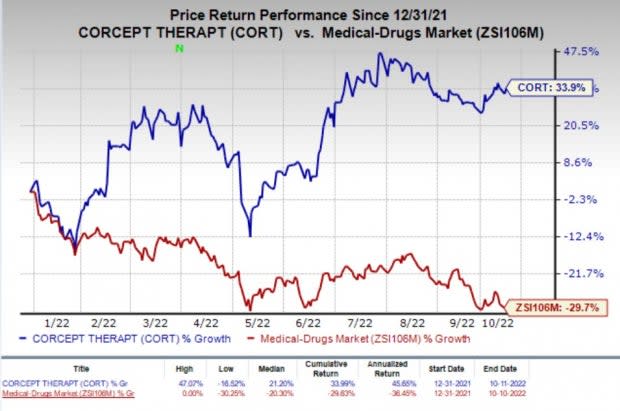
Image Source: Zacks Investment Research
Corcept’s lead pipeline candidate, relacorilant, is being evaluated in the phase III GRACE study to treat Cushing’s syndrome. A new drug application for relacorilant is expected to be submitted in the second half of 2023.
Relacorilant is also being investigated in phase III of the GRADIENT study in patients whose Cushing’s syndrome is caused by adrenal adenoma.
A phase Ib study is evaluating relacorilant in combination with Merck’s MRK PD-1 checkpoint inhibitor, Keytruda (pembrolizumab), for treating patients suffering from adrenal cancer along with cortisol excess.
Merck’s biggest revenue generator, Keytruda, is approved for treating several types of cancer indications. MRK continues to study Keytruda for addressing more cancer indications.
Relacorilant, in combination with nab-paclitaxel, is also being evaluated in the phase III ROSELLA study for the treatment of patients with platinum-resistant ovarian cancer.
Successful development of its candidates remain key to long-term growth at Corcept, given the lucrative market that it targets.
Zacks Rank & Stock to Consider
Corcept currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the same sector is Phio Pharmaceuticals Corp. PHIO, carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Phio Pharmaceuticals’ earnings per share estimates have increased 43.8% for 2022 and 13% for 2023 in the past 60 days.
Earnings of Phio Pharmaceuticals surpassed estimates in three of the trailing four quarters and missed on the other occasion. PHIO delivered an earnings surprise of 21.32%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
Phio Pharmaceuticals Corp. (PHIO) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
